Properties of Ionic Solids
1/35
Earn XP
Description and Tags
Vocabulary flashcards summarizing key terms and properties related to the structure, bonding, and behavior of ionic solids.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
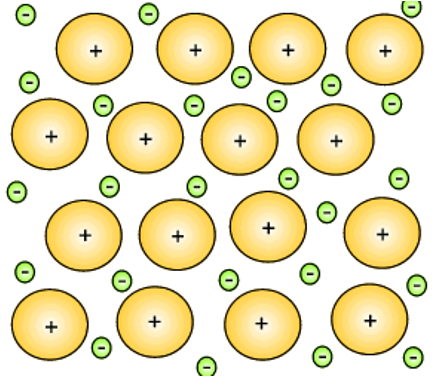
Ionic Solids
A 3D lattice of alternating cations & anions held together by strong ionic (electrostatic) bonds.
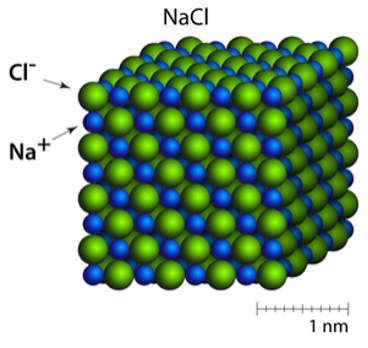
Ionic Bond (Electrostatic Attraction)
Strong force of attraction between oppositely charged ions that holds an ionic lattice together.
Melting Point + Boiling Point (Ionic Solids)
High - ions are held together by very strong ionic bonds (electrostatic forces), which means lots of energy is required to break the bonds. (W)
Electrical Conductivity – Ionic Solids
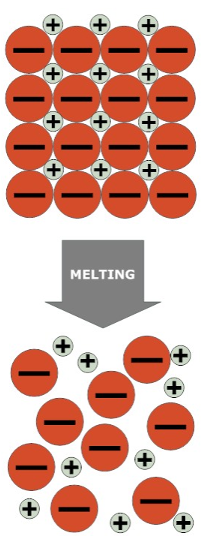
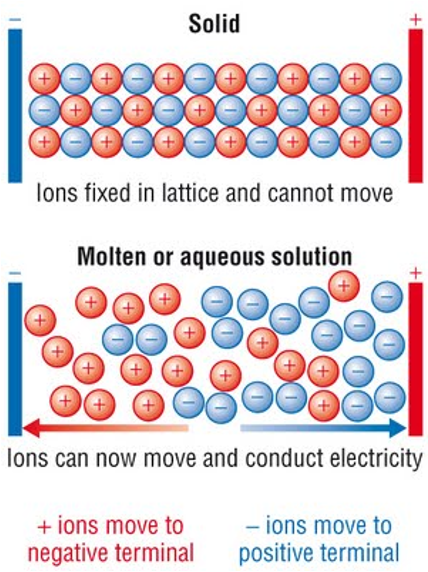
Doesn’t conduct electricity when solid because ions are in fixed positions and cannot move
Electrical Conductivity – Molten/Aqueous Ionic Solid
Conducts electricity because ions are free to move and carry charge.
Hardness (Ionic Solids)
Hard due to the strong ionic bonds between the ions
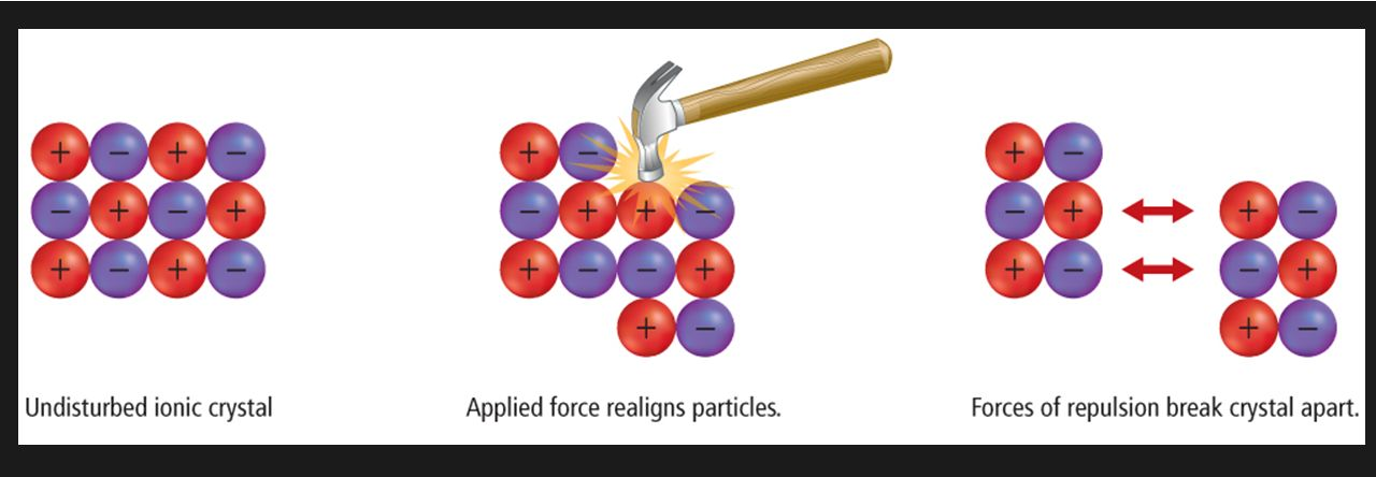
Brittleness (Ionic Solids)
Brittle - The ions are in fixed positions therefore if a force is applied, the ions are moved out of position, like charges lineup and the repulsive forces push the ions apart and the crystal breaks.
Solubility of Ionic Solids in Polar Solvents
yes due to the strong interactions between solvent molecules and the ions, which can disrupt the ionic lattice.
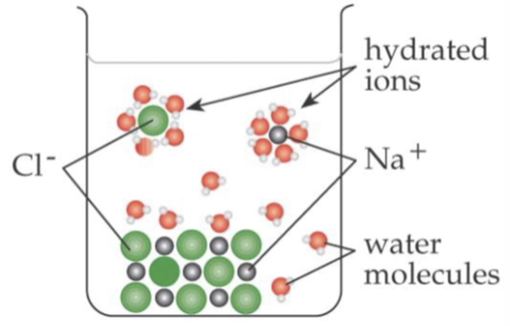
Hydrated Ion
An ion surrounded by and interacting with water molecules in solution.
Electrostatic Forces
Forces of attraction or repulsion between charged particles; in ionic solids, these forces create strong bonding between cations and anions.
Molecular Solids
Discrete molecules held together by weak intermolecular forces.
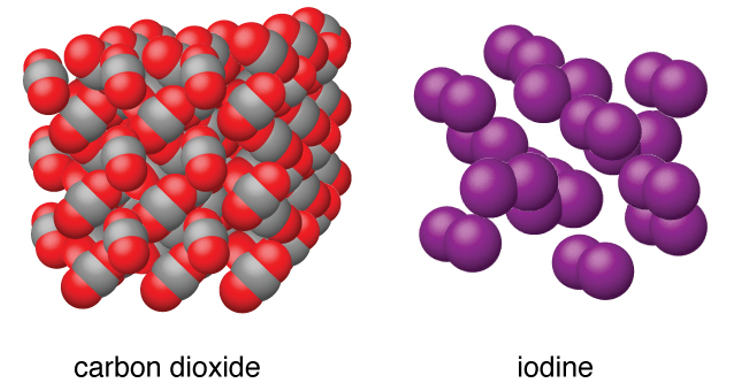
Melting Point and Boiling Point (Molecular Solids)
Low - molecules are held together by weak intermolecular forces, which means little energy is required to break the bonds
-The molecules themselves are not broken up – just moved apart from each other when molten (W)
Electrical Conductivity (Molecular Solids)
Doesn’t conduct electricity as molecular solids do not contain mobile charged particles.
Solubility (Molecular Solids)
Depends on the polarity of the molecular solid and polarity of solvent:
for a substance to dissolve, the solute to solvent attractions must be stronger than the forces of attraction within the solvent and within the solute.
Metals
3D lattice of metal cations surrounded by a sea of delocalised valence electrons. The atoms are held together by non-directional, strong metallic bonds (between the delocalised electrons and the cations).
Characteristics (Metals)
Metals are malleable and ductile as they have non-directional metallic bonds. It doesn’t matter how the particles are positioned as long as they are surrounded by electrons
particles can move past one another / substance can change shape without disrupting the bonding
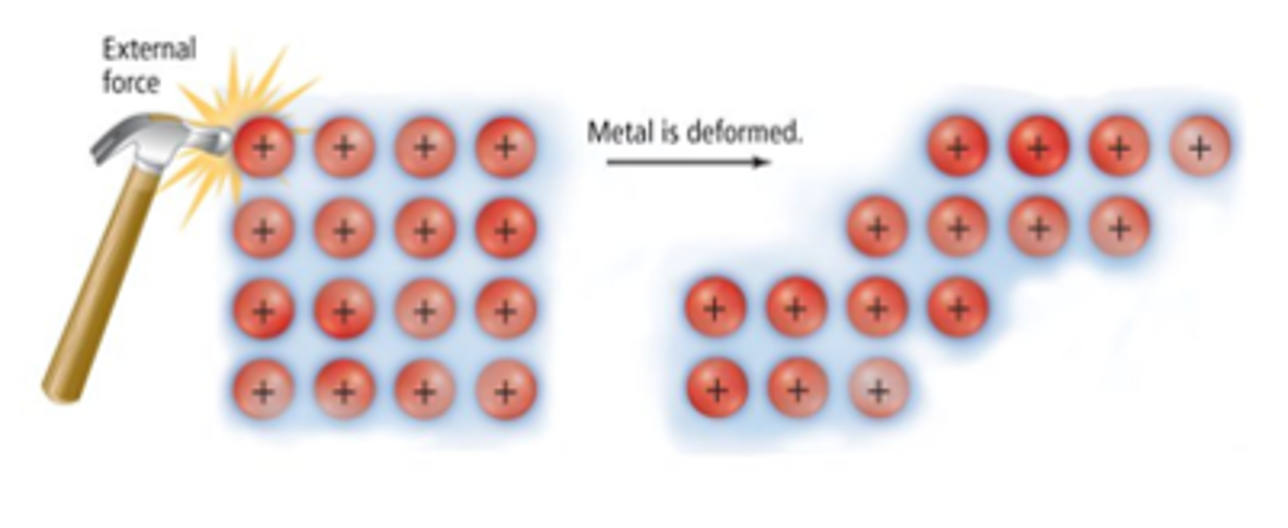
brittleness - metals
Due to the non-directional nature of the metallic bonds, particles can move past one another / substance can change shape without disrupting the bonding (still experiencing the strong electrostatic forces), thus copper can be stretched into wires.
Electrical Conditivity (Metals)
Both metal solids and liquids have high electrical conductivity due to the presence of delocalised electrons that can move freely, allowing the flow of electric current.
●Charge is carried by the delocalised electrons which can move throughout the metallic lattice.
Melting and Boiling Points (Metals)
Varied - Metallic bonds tend to be strong, which means lots of energy is required to break the strong metallic bonds. Hence, the melting and boiling points are high (with exceptions).
Covalent Networks
3D array of atoms held together by covalent bonds
Graphite
2D layers of carbon atoms covalently bonded to three other carbon atoms.
Three of carbon's valence electrons are involved in the bonding. The fourth is delocalised between the layers.
The layers are held together by weak forces.
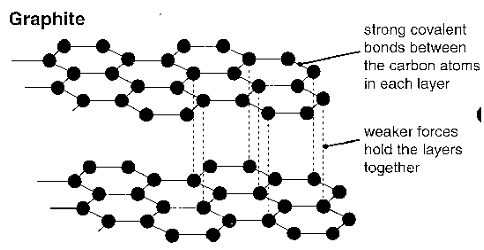
Electrical Conductivity (graphite)
Graphite is a 2D-covalent network substance. It consists of layers of carbon atoms, bound in hexagonal rings by strong covalent bonds. Each carbon is bonded to 3 others.
This means there are (delocalised) valence electrons free to move throughout the structure, allowing graphite to conduct electricity.
To be able to conduct electricity, there needs to be mobile/free moving charged particles. Graphite, C(s), is an extended covalent network solid. Each carbon atom is covalently bonded to 3 other carbon atoms in hexagonal layers. This leaves one delocalised electron per carbon atom that is mobile and able to carry a charge, so graphite conducts electricity.
conducts electricity due to the delocalised electrons which are free to move and carry charge through the network (The only non-metallic element, and the only covalent substance that does)
Characteristics (Graphite)
Slippery - can be used as a lubricant. The weak forces between the layers mean they can easily slide over each other
Diamond
Diamond - 3D array of carbon atoms covalently bonded in a tetrahedral arrangement.
Diamond is made up of a 3D-network of carbon atoms covalently bonded to each other (in a tetrahedral array). Each carbon is bonded to 4 others. This means there are no valence electrons free to move throughout the structure, and diamond does not conduct electricity.
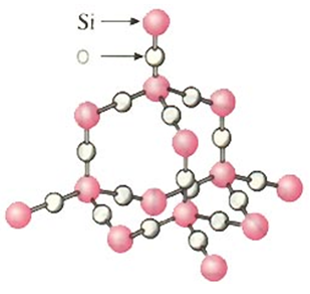
Silica (SiO2)
Silica - 3D array of oxygen and silicon atoms covalently bonded
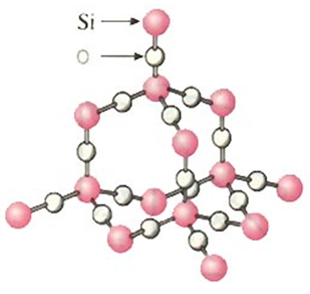
Hardness (Diamond and Silica)
Diamond and silica are very hard due to the strong covalent bonds between the atoms.
Melting and Boiling Points (D+S)
Giant covalent network solids have very high melting point due to the strong covalent bonds between the atoms. (intramolecular?)
●Graphite melts at 3600 oC
Diamond sublimes at 5000oC
Electrical Conductivity (D+S)
None as there are no charged particles.
Solubility (Giant Covalent Networks)
Do not dissolve in water due to the strong covalent bonds between the atoms.
Solubility of Ionic Solids in Non Polar Solvents
Iower solubility than in polar solvents because polar solvents can effectively solvate the ions of ionic compounds, whereas non-polar solvents cannot overcome the strong electrostatic forces holding the ionic lattice together.
Non-polar solvents cannot form permanent ion–dipole attractions. At best, only weak induced dipole–ion interactions occur, which are not strong enough to overcome the strong electrostatic forces holding the ionic lattice together.
Silica
A network of silicon and oxygen atoms, where each silicon atom is bonded to four oxygen atoms, and each oxygen atom is bonded to two silicon atoms, forming a three-dimensional structure.
non polar
no electronegativity difference between the bonded atoms. the electrons are shared equally between the atoms, resulting on no partial charges on either atom
melting point difference conclusion
difference in melting point of substances is due to the differences in the strength of the attractive forces between particles