Johns Hopkins University - Organic Chemistry Lab Exam 2 (Labs E-F, plus A-D from before)
1/96
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
97 Terms
Diels-Alder Reaction
A cycloaddition reaction between a diene and a dienophile to form a cyclic product with 1 new double bond
Cycloaddition reaction
1,4 addition of a 1,3 diene (conjugated diene) and an alkene (dienophile). forms a new 6-membered ring.
Cycloaddition/Diels-Alder new bonds
- 3 pi bonds break between the reacting diene/dienophile
-2 new sigma bonds and 1 new pi bond in the product
(sigma bonds between carbons 1 and 6, and 4 and 5)
(pi bond between carbons 2 and 3)
Reactants in Diels Alder
Diene (1,3-diene, conjugated) and a dienophile (alkene)
Characteristics of a Diels-Alder Reaction
1. Thermal cycloaddition rxn (initiated by heat)
2. Concerted rxn (bond breaking & forming occur in a single step)
3. 3 pi bonds break
4. 2 new C-C sigma bongs and 1 new pi bond formed
5. Forms new 6-membered rings
2 conformations of Dienes
s-cis (double bonds are cis) and s-trans (double bonds are trans)
ONLY S-CIS IS REACTIVE IN DIELS-ALDER
Reactivity of Dienes
Diene must be in s-cis conformation for Diels-Alder reaction to occur
- both ends of the conjugated diene must be closed to the pi bond of the dienophile for the Diels-Alder reaction to occur
Reactivity of Dienophiles
Electron withdrawing groups makes dienophiles more electrophilic --> more reactive
Least to Most reactive:
H2C=CH2 < H2C=CHZ < ZHC=CHZ
Stereospecificity of Dienophile
When a Diels-Alder reaction involves substituted dienophiles, the stereochemistry about the double bond in the dienophile is retained!
- cis-substituted dienophile produces cis-substituted cyclohexene (cis product - meso compounds)
- trans-substituted dienophile produces trans-substituted cyclohexene (trans product- enantiomers)
Variac
Variable voltage transformer, regulates heat transferred to heating mantle. plug under the fume hood.
Purpose of refluxing a reaction
To heat a reaction mixture at its boiling temperature (the boiling point of the solvent used in the reaction) to form the product, without losing any material inside the reaction flask.
Advantages of a reflux
- maintains a constant temperature in the reaction flask
- reaction is able to reach equilibrium with minimal evaporation of material inside the reaction flask
Cheletropic elimination
forms a conjugated molecule. pi bond and 2 sigma bonds break and 2 new pi bonds form
Cheletropic elimination advantages for Lab E
1. Non-hygroscopic solid (does not absorb moisture)
2. Not a flammability hazard
3. Excess 1,3-butadiene and SO2 are gases at room temp and are distilled out during reflux
Overall reaction for Lab E
1. Butadiene sulfone (3-sulfolene) --heat/xylenes--> s-cis-1,3-butadiene
2. s-cis-1,3-butadiene + Maleic anhydride --heat/xylenes--> 4-cyclohexene-cis-1,2-dicarboxylic anhydride
What is xylenes used for in Lab E?
refluxing solvent
Why can't we directly use s-cis-1,3-butadiene in Lab E?
Because it is a gas at room temp and hard to work with. So we use solid butadiene sulfone to generate s-cis-1,3-butadiene in situ so that it can immediately react with maleic anhydride in a Diels-Alder reaction
What happens to sulfur dioxide in Lab E?
the SO2 vapors will climb through the condenser as a white smoke and are distilled out during reflux
What is the purpose of the heating mantle?
to ensure efficient heat transfer. made of insulated fiberglass, supports the base of the RB flask
What kind of flask do we use in Lab E?
a 50 mL round bottom flask
Safety considerations for Lab E
- RB flask NOT on surface of stirrer - need mantle in between because otherwise the organic solvent would boil away, which would ruin the reflux and is a flammability hazard
- cords wrapped around metal bar so it is secure and doesn't flop around
- heating mantle plugged into VARIAC - if plugged into the regular outlet, the heat is unregulated and everything can evaporate, which is bad
- if mantle fiberglass fabric is cracked or the cord is frayed - do not use!
Where do we plug in the heating mantle?
The Variac! never into the regular outlet
Which direction does water flow in the condenser and where does it enter and leave?
Flows up the condenser. Cool water enters the bottom and leaves at the top.
CHWS
chilled water supply (bottom arm)
CHWR
chilled water return (top arm)
How does the water condenser work?
Once the solution reaches the boiling point of the solvent, the solvent vaporizes and vapors climb up the inner hollow tube of the condenser. Water flows up the outer jacket of the condenser. The water cools down the vapor, which causes the vapors to condense back in the reaction flask (forced it back down into the flask because of water in the outer jacket)
- no solvent lost! volume maintained through condensation/evaporation
Where do the vapors go in the condenser?
Up the inner hollow tube
Where does the water go in the condenser?
water flows up in the outer jacket of the condenser
Where do the gases formed in the reaction escape?
gases escape through the inner hollow tube and are vented through exhaust lines inside the fume hood
keep fume hood closed to prevent gases from escaping the hood
What do we put in the reaction flask for Lab E?
we put all of the reactants in at the beginning because we will first do an in situ synthesis, which will immediately be followed by a Diels-Alder reaction.
we add:
- 2.7g of butadiene sulfone
- 1.5g maleic anhydride
- 4 mL xylenes
Why do we need to turn off stirring to check for boiling?
stirring can create bubbles and mislead you to think the solution is boiling
What do we do after the solution is boiling?
remove the foil covering
What do we see climbing the condenser in Lab E?
solvent vapors
white smoke (SO2 vapors)
Make sure the condenser is always _____ to the touch
cold
What happens if the condenser is not cold to the touch?
This can be caused by attaching the CHWS and CHWR in the wrong place. Hot condenser means reflux is not happening and this could cause all the solvent to boil away, which is a flammability hazard.
Limiting reagent and excess reagent in Lab E
Limiting reagent = maleic anhydride
Excess reagents = 1,3-butadiene and SO2 (distilled out as gases during reflux)
How long is the reflux for Lab E?
30 mins
What do we do after refluxing the solution in Lab E?
cool to flask and then add 9mL xylenes to the RB flask with a pasteur pipette, then warm the flask in a warm water bath for 2 mins
Why do we add xylenes to the reaction flask after refluxing? What do we use to add the xylenes?
to dilute the mixture in the RB flask. add using a pasteur pipette
Why do we decant the supernatant fluid in Lab E?
To avoid impurities. a brown sludge can form, so decanting leaves this in the RB flask so that we can just have the supernatant in the beaker without the impurities
Why do we add petroleum ether in Lab E, how much do we add, and what do we use to add it?
We add 10mL of petroleum ether using a pasteur pipette in order to help the Diels-Alder product crash out of solution. The product is much more soluble in xylenes than in pet. ether, so we add low boiling pet. ether to help it precipitate.
What do we do to separate the DA product from the solvent in Lab E?
Vacuum filter - use spatula to scoop the caked product into the funnel. then after filtering, scoop it out of the funnel w a spatula
Vacuum filter paper in Lab E
DO NOT WET THE FILTER PAPER WITH H2O!!! the reaction was done in organic solvent, so we don't want to introduce an aqueous solvent
Appearance of Diels Alder product
product: 4-cyclohexene-cis-1,2-dicarboxylic anhydride
wet product: solid white, yellowish crystals
put onto watch glass to dry and take a tiny spatula's tip worth and put into a vial for IR spectroscopy
IR spectroscopy
used to detect functional groups present
IR absorptions
due to stretching and bending of covalent bonds in molecules
Regions in IR spectroscopy
Functional group (diagnostic region): determines the functional groups present
Fingerprint region: used for structure elucidation by spectral comparison
Bonds and frequency
Stronger bonds = higher frequency
lighter atoms = higher frequency
higher frequency = higher energy
What is Fisher esterification used for?
reliable method to synthesize esters on an industrial scale
Fisher esterification general reaction
carboyxlic acid + alcohol --acid catalyst--> ester + H2O
How do we push the esterification reaction in the forwards direction (since it's an equilibrium?)
can either:
- use an excess reactant (like in Lab F)
- remove water as it forms
Esters
RCOOR
- fruity odors
- used as flavoring agents, also used in perfume industry
General method for preparing esters
reflux mixture of carboxylic acid and an alcohol in the presence of an acid catalyst, like concentrated H2SO4 of HCl
- reflux
- extraction
- distillation
Overall reaction for Lab F
isopentyl alcohol (3-methyl-1-butanol) + glacial acetic acid --(conc. H2SO4)--> isopentyl acetate (banana oil) + H2O
Glacial acetic acid
the anhydrous form (no H2O) of acetic acid
- called glacial because it forms needle-shaped crystals when frozen
- excess reactant in the reaction (most economical)
- BP 118 degrees C
Glacial acetic acid hazards
Flammable (2), Health (3), Corrosive
Sulfuric acid hazards
Corrosive, Health (3)
Boiling stones
- use to avoid bumping during reflux, maintains even boiling
- never reuse boiling stones
- scoop them out with a spatula and depose of in biohazard box
Reaction flask used in Lab F
100 mL round bottom flask
What temperature is the reflux carried out at for Lab F?
117-118 degrees C, the boiling point of glacial acetic acid
How long is the reflux time for Lab F?
50 mins
What is the color of the solution after refluxing in Lab F?
Deep purple-red color
Size of sep funnel used in Lab F
125 mL sep funnel
What type of funnel do we use to pour the solution into the sep funnel?
pyrex funnel (long stem)
do NOT let boiling stones go into the funnel! will clog it!
Separation of layers in sep funnel after adding 50 mL water
- top layer = organic layer, contains glacial acetic acid solvent, excess isopentyl alcohol, regenerated acid catalyst, and of course the product isopentyl acetate
- bottom layer = aqueous layer, contains the water
Addition of NaHCO3 to sep funnel
- fizzing due to CO2 formation
- neutralizes leftover glacial acetic acid in solution
Addition of brine to sep funnel
begins drying process by draws out large amounts of H2O in organic layer
Top organic layer
-drain into 125 mL Erlenmeyer flask
- add anhydrous CaCl2 drying agent
Anhydrous CaCl2
drying agent, pulls out H2O
- add using a spatula until it no longer clumps together and is free flowing
Gravity filter
traps CaCl2 in filter paper and collected liquid isopentyl acetate in RB flask
Why do we do a simple distillation in Lab F?
- isopentyl acetate product may contain unreacted isopentyl alcohol
- we don't do a fractional distillation with a Vigreux column because that would cause us to lose a lot of product - instead, we do a simple distillation
Parts of a simple distillation
- 3-way distillation adapter
- 2 blue keck clips
- thermometer adapter
- vacuum adapter
- thermometer
- labeled vial for product
- 2 new boiling stones
Ring stands in the simple distillation
- 1 clamped around 25 mL RB flask
- 1 clamped around water condenser
Flask used for simple distillation
25 mL RB flask
3-way adapter arms connections
- side arm: water condenser
- bottom arm: neck of the RB flask
- top arm: thermometer and thermometer adapter
Blue keck clips
connect glass on glass joints
How many boiling stones go into the flask during simple distillation in Lab F?
2 boiling stones
Thermometer in simple distillation
- alcohol thermometer is poorly calibrated at room temp, so we collect over a bp range of 125-138 degrees C even though the lit bp of isopentyl acetate is 142 degrees C
- thermometer red bulb MUST BE COMPLETELY BELOW THE SIDE ARM OF THE 3 WAY ADAPTER!!!
Distillation in Lab F
- cover flask for 5 mins until reaches boil
- collect material between 125-138 degrees C
- vapor start condensing on thermometer bulb
- then, vapors go through water condenser and are cooled by the cold water and become a clear colorless liquid
Appearance of final product in Lab F
Final product = isopentyl acetate aka banana oil: clear, colorless liquid
Why don't we distill all of the liquid in the reaction flask?
leave 1-2 mL of liquid in RB - don't distillate to dryness because this will char the flask and make it very difficult to clean. creates pyrolytic tars!
Simple Distillation
- technique used to separate 2 or more liquids
- used to separate 2 or more liquids below 150 degrees C at 1 atm from nonvolatile impurities or another liquid that boils at least 25 degrees C higher than the first
- liquid is vaporized then recondensed back to a liquid (distillate) and collected in a receiving flask
Industry distillation
widely used in industry. separates oils into different fractions based on bp differences
Vacuum distillation
used for compounds that boil at too high a temp or decompose near their bp. under vacuum, can be distilled at temps lower than their atmospheric bp. use only for compounds with bp>200 degrees C
Fractional distillation
- use to separate compounds that are soluble in each other and boil at less than 25 degrees C from each other at 1 atm
- use Vigreux column with indentations - provides more surface area over which a number of separate liquid-vapor equilibria can occur
- efficiency = expressed by number of plates
- each cycle causes the vapors to become more enriched in the more volatile/low boiling liquid
Factors affecting boiling points
1. Size (molecular weight)
- larger = higher bp
2. Intermolecular interactions
- more/stronger interactions = higher bp
Boiling point
temperature at which the vapor pressure = external pressure
How much does the bp increase per additional carbon?
30-40 degrees C
Clausius-Clapeyron equation
an equation that displays the exponential relationship between vapor pressure and temperature
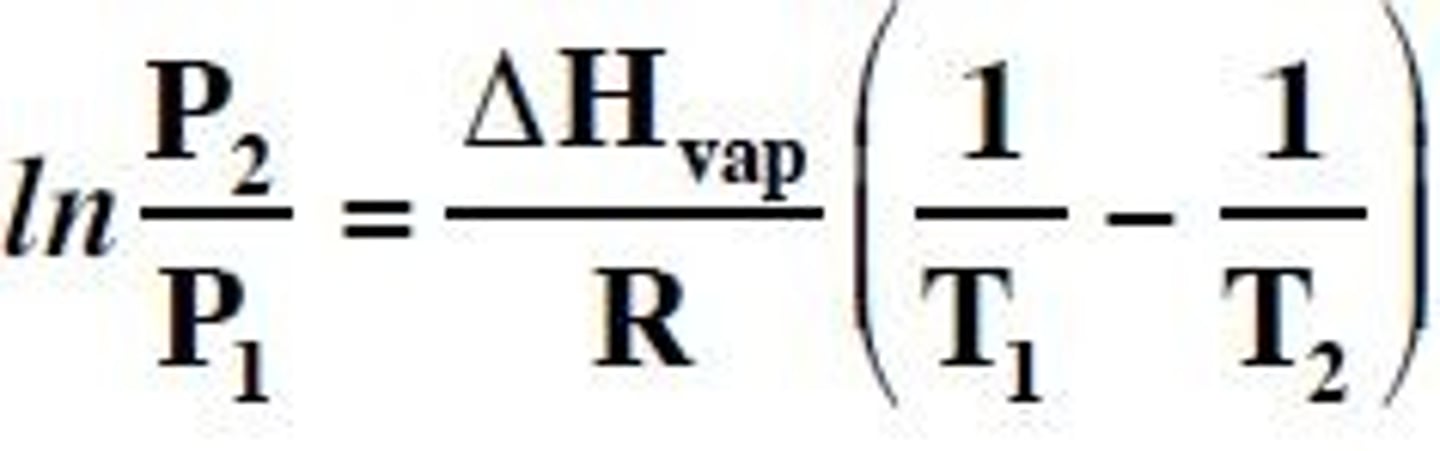
Normal boiling point
defined as temp at which a liquid's vapor pressure = 1 atm
more molecules vaporize at higher temps
Thermometer bulb position
COMPLETELY below the side arm of the 3-way adapter
read the thermometer when the material starts collecting, not when the solution starts boiling
When do we read the thermometer?
read when the material starts collecting, not when the solution starts boiling
Partition coefficient
K=Solubility of solute in organic layer/solubility of solute in aqueous layer
Excess reactant in Lab F
glacial acetic acid
Why do we add NaHCO3 in Lab F
adding NaHCO3 deprotonates the acetic acid, which is a carboxylic acid (pka=5, can be easily deprotonated), into sodium acetate. Sodium acetate is soluble in H2O, so it's drawn into the aqueous layer and we can remove it when we drain the bottom layer (and then we are just left with isopentyl acetate and maybe a little unreacted isopentyl alcohol). This reaction produces CO2, so must vent when shaking!!
In the Diels-Alder reaction, when we do a vacuum filtration, what do we not wet the filter paper with?
Water
The filtrate collected during the Diels-Alder reaction is disposed of into ____________________.
CHO non-halogenated container