Levels of protein structure
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
18 Terms
What are the four levels of protein structure?
Primary structure – sequence of amino acids in the polypeptide chain.
Secondary structure – local folding into α-helixes or β-pleated sheets via hydrogen bonding between -NH groups and -CO groups.
Tertiary structure – further folding into a 3D shape due to R group interactions.
Quaternary structure – multiple polypeptide chains (subunits) joined together.
Extra (OCR detail): Some proteins also contain prosthetic groups (non-protein components), e.g. haem in haemoglobin.
What defines the primary structure of a protein?
The sequence of amino acids in the polypeptide chain, joined by peptide bonds (covalent).
It is determined by the sequence of bases in DNA, which codes for the sequence of amino acids during translation.
Why is the primary structure important?
Even a single change in the amino acid sequence can alter how the chain folds, changing the protein’s shape and function.
What bonds hold the secondary structure together?
Hydrogen bonds between the carbonyl (C=O) group of one amino acid and the amine (N–H) group of another within the protein backbone.
What are the two main forms of secondary structure?
α-helix – coiled shape stabilized by hydrogen bonds every 4th peptide bond.
β-pleated sheet – sheet-like structure where parallel regions are held by hydrogen bonds.
Which types of proteins often have secondary structure as their main form?
Fibrous proteins, e.g. collagen, keratin.
How can secondary structure be disrupted?
By high temperature or pH changes, which break hydrogen bonds.
What determines the tertiary structure of a protein?
The 3D folding of a polypeptide due to interactions between R groups (side chains) of amino acids.
What bonds are involved in tertiary structure?
Hydrogen bonds (between polar R groups – weakest but most common)
Ionic bonds (between oppositely charged R groups, e.g. –NH₃⁺ and –COO⁻), can be disrupted by pH changes
Disulphide bridges (strong covalent bonds between cysteine amino acids where the sulfur atom in one cysteine amino acid bonds to the sulfur atom on another)
Hydrophobic interactions (between non-polar R groups inside the protein causes them to clump together which pushes hydrophillic R groups to the outside which affects how the protein folds up into its final structure
Which of these bonds is the strongest?
Disulphide bridges are the strongest (covalent) but occur less frequently.
How can each type of bond be broken?
Hydrogen: by heat or pH change
Ionic: by pH change
Disulphide: by reducing agents (oxidation/reduction reactions)
Hydrophobic: by detergents or heat
Why does tertiary structure determine function?
Because the specific 3D shape determines how the protein interacts with other molecules (e.g. enzyme active site or receptor binding).
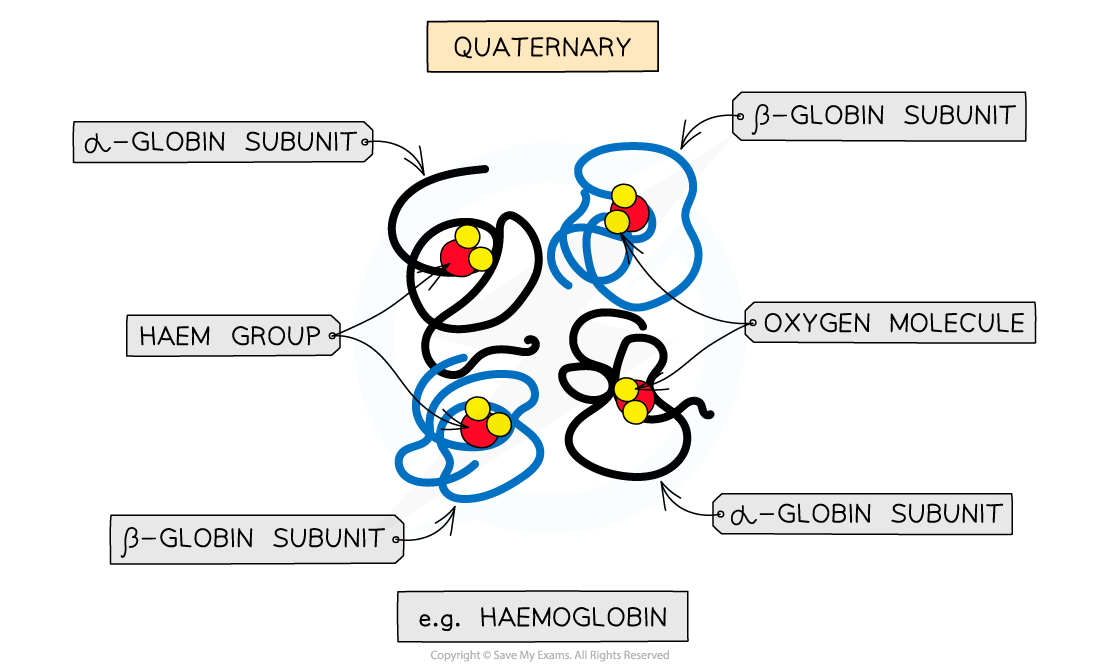
What is quaternary structure?
The structure of proteins with two or more polypeptide chains (subunits) bonded together, sometimes with non-protein prosthetic groups.
do all proteins have a quaternary structure?
no as some are only made of one polypeptide chain
Give an example of a quaternary protein.
Haemoglobin – composed of four polypeptide subunits (2α + 2β) and a haem group (prosthetic group containing iron).
What holds subunits together in quaternary structure?
The same types of interactions as in tertiary structure (hydrogen, ionic, hydrophobic, disulphide).
What is the key difference between hydrogen bonds in secondary and tertiary structures?
Secondary: Between the amine and carboxyl groups (protein backbone).
Tertiary: Between R groups of amino acids.
what affects quaternary structure of proteins
Quaternary structure depends on the tertiary structure of the
individual polypeptides, and so is influenced by all these
bond types ( such as ionic bonds, disulphide bonds etc.)