Chapter 7 Trends in the Periodic Table
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
atomic radius ____ along a period
decreases
atomic radius ___ down a group
increases
atomic radius decreases
increase in nuclear effective charge
no increase in screening effect
atomic radius increases
new energy level
screening effect
ionisation/electronegativity ___ along group
increases
ionisation/electronegativity ___ down a group
decreases
ionisation/electronegativity increases
increasing effective nuclear charge
decreasing atomic radius
ionisation/electronegativity decreases
increasing atomic radius
screening effect
alkali metals
reactivity increases down a group, increase in atomic radius screening effect
reaction in water
base and hydrogen gas formed, red litmus paper to blue
halogens
reactivity decreases down group due to electronegativity decreasing
Elements in the same period
• Have the same number of shells (energy levels) outside their nucleus
Elements in the same group
• Have the same number of electrons in their outer shell (energy level)
Group 1 (I) – The Alkali Metals
All the members:
• Have a valency of 1
• Highly reactive
• Become more reactive going down the group
• Are not found freely in nature – only found in compounds with other elements
Properties of the alkali metals:
(1) Soft metals
(2) Low melting points and boiling points
(3) Have low densities
Why are the alkali metals highly reactive?
- They do not satisfy the octet rule – they only have one electron in their outer shell
- In order to satisfy the octet rule they readily lose this electron when they react and form a monopositive ion (+1)
Why does the reactivity of the alkali metals increase going down group I
- The atomic radius increases
Reactions of the alkali metals: (1) Reaction with oxygen
• The alkali metals react rapidly with oxygen to form a metal oxide
• For this reason, they are stored in oil/paraffin
metal + oxygen → metal oxide
Reactions of the alkali metals: 2) Reaction with water
- The alkali metals:
i) Float on water
ii) React vigorously with water
iii) Causes effervescence/fizzing with water
iv) Produce a metal hydroxide and hydrogen gas when reacted with water
metal + water → metal hydroxide + hydrogen
Notes: Metal hydroxides are alkaline – will turn pink with phenolphthalein indicator
To test for Hydrogen gas - Hydrogen gas ignites with a ‘pop
Group 2 (II) – The Alkaline Earth Metals
All the members:
• Have a valency of 2
• Reactive - but not as reactive as the alkali metals
• Become more reactive going down the group
Why are the alkaline earth metals highly reactive?
- They do not satisfy the octet rule – they two electrons in their outer shell
- In order to satisfy the octet rule they readily lose this electron when they react and form a dipositive ion (+2)
Why does the reactivity of the alkali metals increase going down group II
- The atomic radius increases
Reactions of the alkaline earth metals: (1) Reaction with oxygen
• The alkali metals react with oxygen to form a metal oxide
• Not as rapid a reaction as the alkali metals with oxygen
metal + oxygen → metal oxide
Reactions of the alkaline earth metals: (2) Reaction with water
• The alkaline earth metals react with water to form a metal hydroxide and hydrogen gas
• Not as rapid or as vigorous a reaction as the alkali metals with water
metal + water → metal hydroxide + hydrogen
Notes: Metal hydroxides are alkaline – will turn pink with phenolphthalein indicator
To test for Hydrogen gas - Hydrogen gas ignites with a ‘pop’
Transition Elements – the d-block elements
• Transition elements are found between groups II and III in the periodic table
• Transition metals form at least one ion which has an incomplete 3d sublevel
Features of transition elements:
(1) They have variable valencies
Example: Cu+ and Cu2+ (valency of 1 or 2)
(2) They form coloured compounds (not white)
Example: CuSO4 (Copper (II) sulfate) - blue (Cu2+)
Cu2SO4 (Copper (I) sulfate) - brick red (Cu)
(3) They are excellent catalysts
Group 7 (VII) or Group 17 – The Halogens
All the members:
• Have a valency of 1
• Have seven electrons in their outer shell
• Become less reactive going down the group
• Form diatomic molecules – composed of two atoms joined by a covalent bond
Why are the halogens reactive?
- They do not satisfy the octet rule – they seven electrons in their outer shell
• In order to satisfy the octet rule – they gain one electron when they react and form a mononegative ion (-1)
Why does the reactivity of the halogens decrease going down group VII
- The atomic radius increases
What intermolecular forces occur between halogen molecules?
• Van der Waalsforces form between halogen molecules due to being non-polar molecules
Comment on the boiling point of the halogens
• Halogen molecules are non-polar - have weak van der Waals forces between their molecules
• Therefore the halogens have low boiling points
• As the molecule’s molecular mass (Mr) increases, the strength of the van der Waals forces increases
- Fluorine (F2) and Chlorine (Cl2) are gases at room temperature
- Bromine (Br2) is a volatile liquid at room temperature
- Iodine (I2) is a volatile solid at room temperature
Group 8 (VIII) or Group 18 – The Noble Gases
All the members:
• Have a valency of 0
• Have eight electrons in their outer shell - this is a stable arrangement of electrons do not gain, lose, or share electrons – already satisfy the octet rule
• Makes them very inert and safe to use
Examples of using noble gases: Using helium gas in hot air balloons
Using neon gas in lights
Define atomic radius/covalent radius
• Atomic radius is half the distance between the nuclei of two atoms of the same element joined by a single covalent bond
State and explain the trend in atomic radii (covalent radii) across a period of the periodic table
State: Atomic radii values decrease going across a period (atoms get smaller)
Explain: The effective nuclear charge increases - more protons in the nucleus means there is an
increasing attraction between the nucleus and the electrons in the outer shell and the atom is “pulled smaller”
State and explain the trend in atomic radii (covalent radii) down a group of the periodic table
State: Atomic radii values increase going down a group (atoms get larger)
Explain: An additional shell of electrons is added on
Why is establishing an atomic (covalent) radius for the noble gases problematic?
• Atomic radius is half the distance between the nuclei of two atoms of the same element joined by a single covalent bond
• The noble gases satisfy the octet rule – they do not gain, lose, or share electrons so do not form bonds with other elements - they are monatomic
Define electronegativity
• Electronegativity is the measure of relative attraction an atom has for a shared pair of electrons in a covalent bond
State and explain the trend in electronegativity values across a period of the periodic table
State: Electronegativity values increase going across a period (atoms get better at ‘tug of war’!)
Explain:
1. The atomic radius decreases - the pair of electrons in the covalent bond become closer to the positive nuclear charge
2. The effective nuclear charge increases - more protons in the nucleus means there is an increasing attraction between the nucleus and the electrons in the covalent bond
State and explain the trend in electronegativity values down a group of the periodic table
State: Electronegativity values decrease going down a group (atoms get worse at ‘tug of war’!)
Explain:
1. The atomic radius increases - the pair of electrons in the covalent bond become further
from the positive nuclear charge
2. No increase in effective nuclear charge due to an extra shell – electrons in covalent bond are more shielded from the nucleus
Why do the noble gases not have electronegativity values?
• Electronegativity is the measure of relative attraction an atom has for a shared pair of electrons in a covalent bond
• The noble gases satisfy the octet rule – they do not gain, lose, or share electrons so do not form bonds with other elements and so have no electronegativity values
Define ionisation energy
• Ionisation energy is the minimum energy required to remove the most loosely bound electron from an atom or ion
Define first ionisation energy
• First ionisation energy is the minimum energy required to remove the most loosely bound electron from a neutral gaseous atom in the ground state
Representing first ionisation energy in the form of an equation
X → X+ + e-
What unit is used to measure ionisation energy?
• Kilojoules per mole (kJ mol-1)
State and explain the trend in first ionisation energy values across a period of the periodic table
State: First ionisation energy values generally increase going across a period (takes more energy to remove the electron)
Explain:
1. The atomic radius decreases - the electron being removed becomes closer to the positive nuclear charge
2. The effective nuclear charge increases - more protons in the nucleus means there is an increasing attraction between the nucleus and the electron being removed
Important: there are exceptions to this general trend of first ionisation energies going across a period
Notice:
1) Going across a period, first ionisation energy values increase
2) In period 2 and period 3 there are two exceptions to this general increase in first ionisation energy
3) Going from one period to another there is a large decrease in first ionisation energy
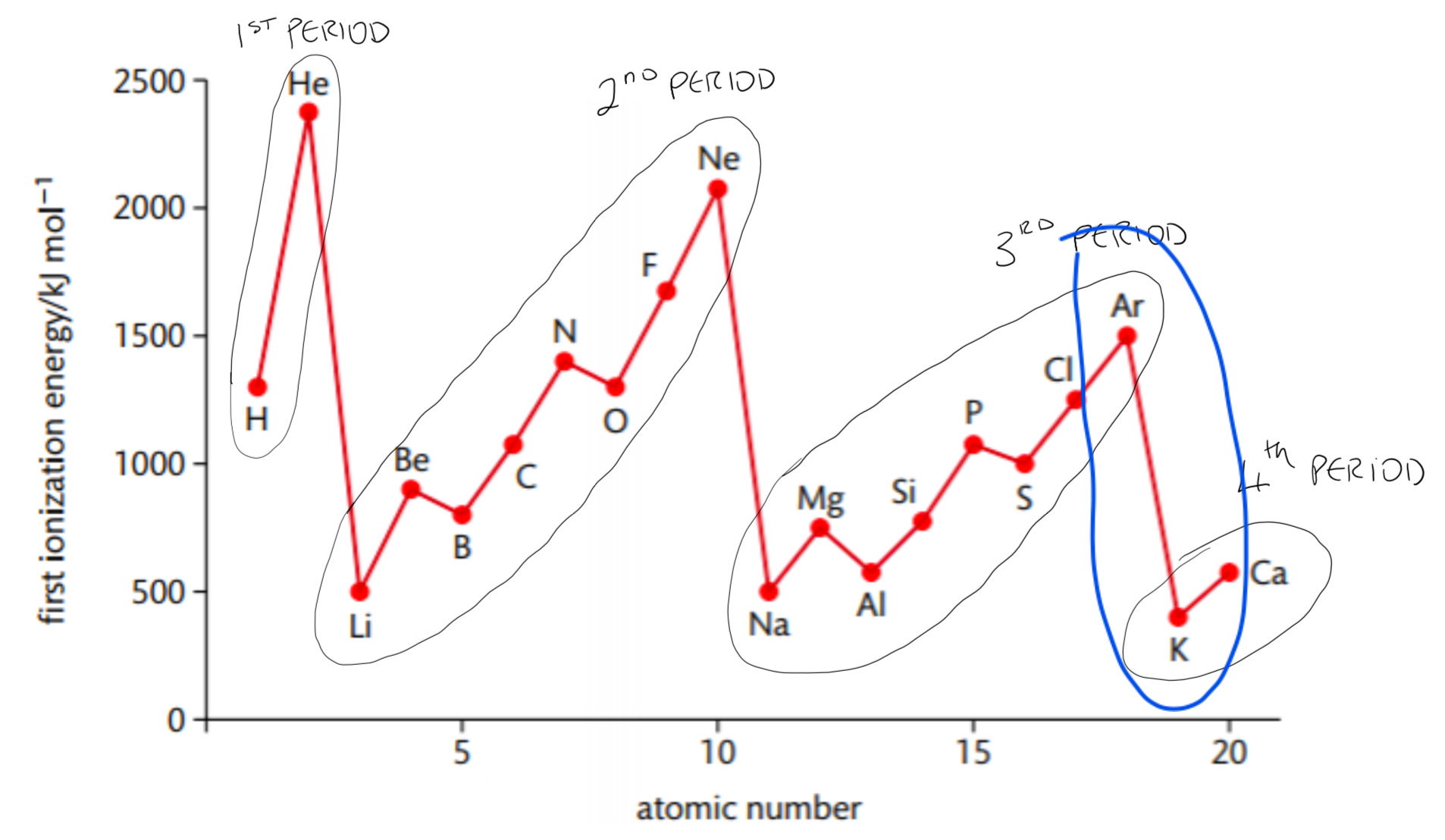
State and explain the trend in first ionisation energy values down a group of the periodic table
State: First ionisation energy values decrease going down a group (takes less energy to remove the electron)
Explain:
1. The atomic radius increases - the electron being removed becomes further from the positive nuclear charge
2. No increase in effective nuclear charge due to an extra shell - most loosely bound electron is more shielded from the nucleus
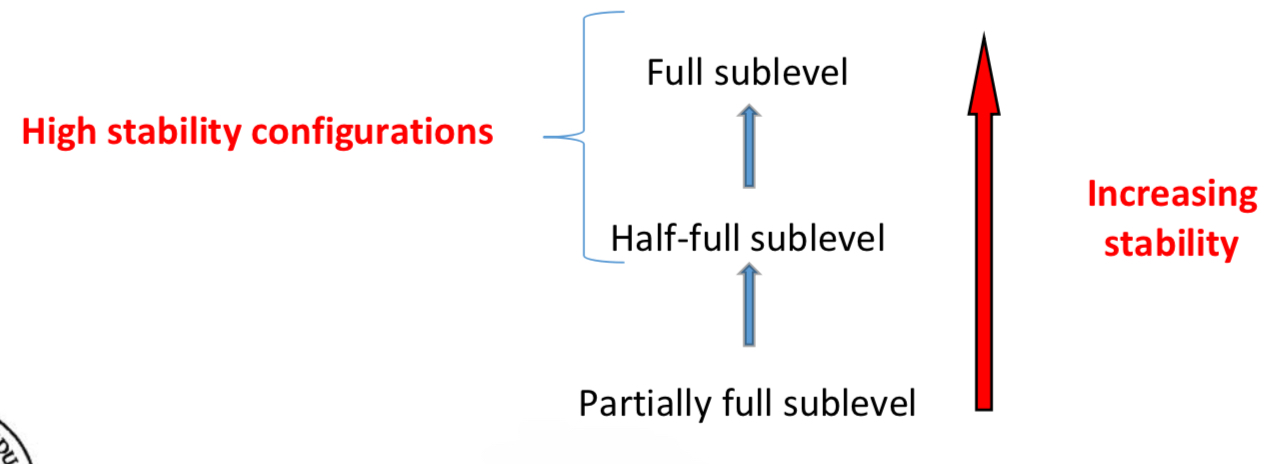
Explaining the exceptions to the general trend in first ionisation energy values across a period
Write out the s, p configuration for the element involved and look at where the first electron isbeing removed from
Electrons being removed from full sublevels, and half-full sublevels will require extra energy as theseare high stability configurations
Explaining the large substantial energy decreases going from one period to another
- An element in the 1st period has their first electron removed from the 1st shell
- An element in the 2nd period has their first electron removed from the 2nd shell
- An element in the 3rd period has their first electron removed from the 3rd shell
➢ It takes substantially less energy to remove an electron from a shell further from the nucleus – this explains why there are large decreases in first ionisation energy going from one period to another
Define second ionisation energy
• Second ionisation energy is the minimum energy required to remove the most loosely bound electron from a positive ion
X+ → X2+ + e-
Give two reasons why the second ionisation energy of an element always greater than the first?
1) The second electron is being removed from a positive ion- the effective nuclear charge has increased so there is a greater attraction between the electron being removed and the nucleus
2) The atomic radius has decreased in the positive ion – the second electron being removed is closer to the positive nuclear charge
Note: If the second ionisation energy of an element is significantly/substantially larger than the first, the second electron is being removed from a shell closer to the nucleus
Looking at successive ionisation energy values for one element
- Questions may be given where the full set of ionisation energy values for a particular element are shown. i.e. the energy values are shown for every electron in the element being removed one by one
- When given successive ionisation energy values for one element, write out the s, p configuration for the element involved and look at where each electron is being removed from
How does looking at successive ionisation energy values for an element provide evidence for the existence of energy levels in atoms?
• When attempting to remove an electron from a new energy level, a substantially higher amount of energy is required.
• Looking at the number of substantial increases in ionisation energy will show how many energy levels are occupied by electrons in the atom