Intro to Immunology- Exam 2
1/135
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
136 Terms
Two classes of molecule used by the adaptive immune system to specifically recognize and respond to antigens:
Antibodies and T cell receptors
Two forms of antibodies
Membrane-bound antibodies on the surface of B lymphocytes that function as antigen receptors
Secreted antibodies that protect against microbes
What are immunoglobins?
antibodies are a type of immunoglobin (Ig)
primary mediator of humoral immunity
produced exclusively by B cells
incredibly diverse and specific in their ability to recognize foreign molecular structures
Where are antibodies found?
secreted forms reside in the circulation, tissues, and mucosal sites
Antigen
a molecule that binds to an antibody or a T cell receptor
Structure of an antibody?
most of the antibody variability is contained within three short regions called the hypervariable regions
different isotypes and subtypes perform different effector functions
Where does the antigen bind to an antibody?
The V (variable) region
What is the Fc region of an antibody?
The effector portion; bind to specific receptors on innate cells to activate phagocytosis or other immune functions
Antibody isotypes
IgA —> mucosal immunity
IgD —> native B cell antigen receptor
IgE —> defense against helminthic parasites, immediate hypersensitivity
IgG —> opsonization, complement activation, antibody-dependent cell-mediated cytotoxicity, neonatal immunity, feedback inhibition of B cells
IgM —> naive B cell antigen receptor, complement activation
How are antibody classes determined?
Antibodies can be divided into classes and subclasses based upon their heavy chain C regions
Which antibody isotypes are made first?
During an immune response, IgM is always produced first
How do antibodies change during the immune response?
Affinity maturation (somatic mutations in variable region)
Change from membrane form to secreted form
Switching of isotypes
Polymorphism
a trait that described
Co-dominance
when two alleles of the same gene are expressed separately to yield different traits in an individual
Syngeneic
when individuals express the same alleles
Allogeneic
when individuals express at least one differing allele
Allele
MHC restriction
A given T cell can only recognize its specific antigen in the context of a specific MHC molecule
MHC haplotype
the set of alleles for a given individual
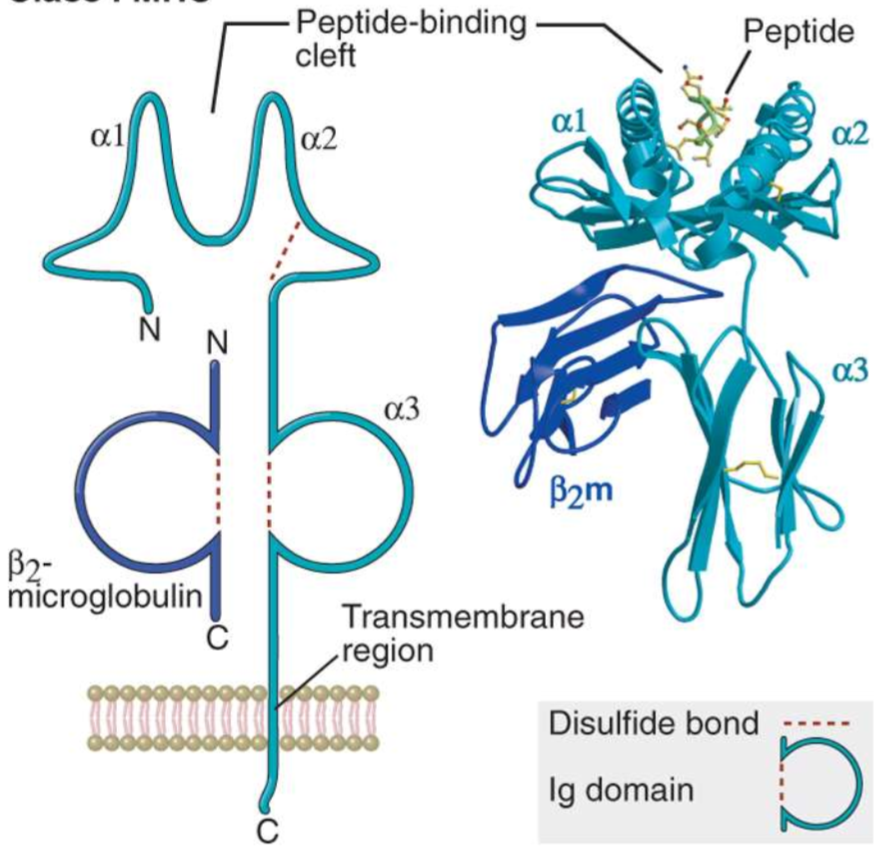
Class I molecule structure
heterotrimer consisting of an alpha chain, 2 beta microglobulin, and a bound peptide
all three must be present for expression on the cell surface
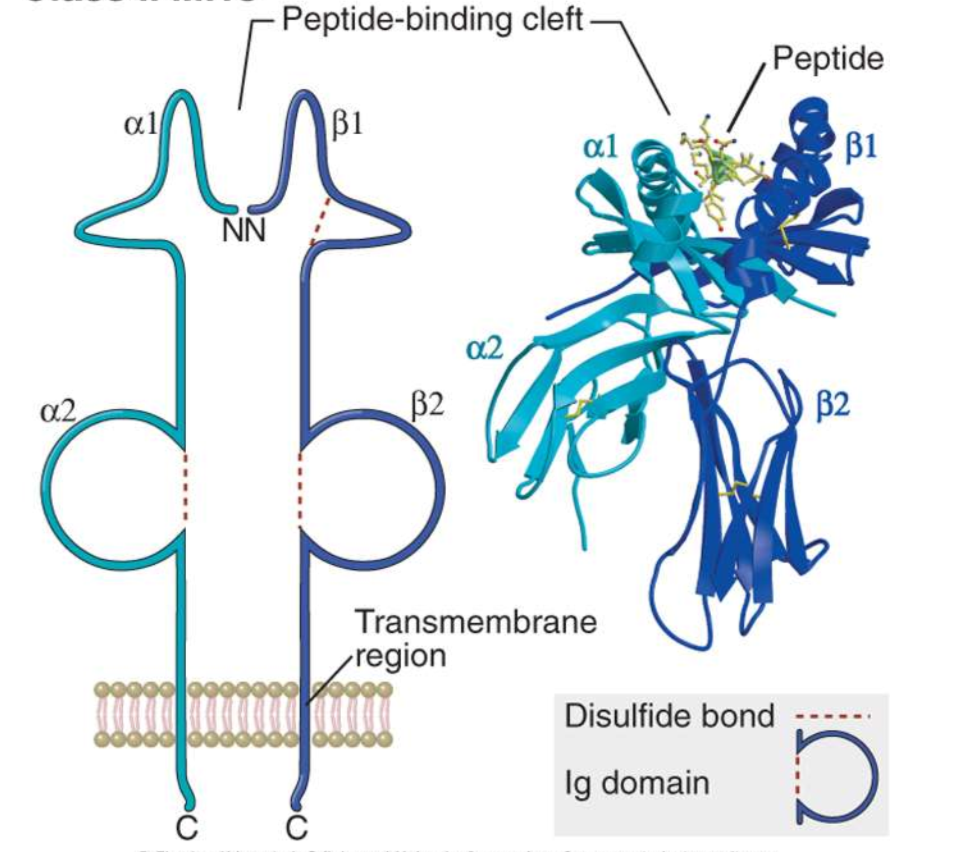
Class II molecule structure
consists of two alpha chains and two beta microglobulin
Characteristics of peptide-MHC interactions
polymorphic residues are located both on the helices and on the floor of the peptide-binding groove
these residues add greatly to the diversity of peptides that can bind to a given MHC molecule
Expression patterns of class I MHC molecules
this class of MHC molecule is constitutively expressed on all nucleated cells
Expression patterns of class II MHC molecules
this class of MHC molecule is expressed only on professional antigen presenting cells
Properties of antigens recognized by T cells
most T cells recognize antigens and no other molecules, since only peptides bind to MHC molecules
T cells recognize cell-associated and non-soluble antigens
CD4+ and CD8+ T cells preferentially recognize antigens sampled from the vesicular and cytosolic pools, respectively
Role of an antigen-presenting cell in T cell activation
Dendritic Cells: naive T cell activation —> clonal expansion and differentiation into effect T cells
Macrophages: effector T cell activation —> cell-mediated immunity through activation of macrophages
B cells: effector T cell activation —> B cell activation and antibody production (humoral immunity)
What is the function of an adjuvant?
to enhance and modulate the immune response to make it stronger and more efficient
What cells are “professional APCs”?
Dendritic cells
Macrophages
B cells
What is the general process of antigen capture and presentation?
Antigen uptake
Antigen processing
MHC biosynthesis
Peptide-MHC association
Class I pathway of antigen processing
Production of proteins in the cytosol
Proteolytic degradation of proteins
Transport of peptides from cytosol to ER
Assembly of peptide-class I complexes in ER
Surface expression of peptide-class I complexes —> bind to CD8+ T cell
Class II pathway of antigen processing
Uptake of extracellular proteins into vesicular compartments of APC
Processing of internalized proteins in endosomal/lysosomal vesicles
Biosynthesis and transport of class II MHC molecules to endosomes
Association of processed peptides with class I MHC molecules in vesicles
Expression of peptide-MHC complexes on cell surface —> bind to CD4+ T cells
Endosome
organelles inside eukaryotic cells that are important for sorting and transporting materials within the cell, including proteins and lipids
What is CLIP?
Class II-associated invariant chain peptide
binds to the peptide-binding groove of MHC class II and remains there until the MHC receptor is fully assembled
plays a critical role in regulating MHC class II folding, transport, and peptide occupancy
HLA-DM
human leukocyte antigen DM
non-polymorphic MHC class II molecule that plays a key role in presenting peptides from outside the cell and interacting with CD4+ T helper cells on immune cells such as B cells and APCs
What is ubiquitin and what does it do?
it is a protein modifier that attaches to and “tags” proteins to mark them for degradation via proteasomes
Proteosome
a protein complex that breaks down unwanted proteins in a cell
What is TAP and what does it do?
transporter associated with antigen processing
a protein that is essential for peptide delivery from the cytosol into the lumen of the ER, where these peptides are loaded on MHC class I molecules
What is the general model of ligand-receptor signaling?
“signaling from the cell surface causes something to happen”
ITAMs
“immunoreceptor tyrosine-based activation motif”
sends an activating signal into the cell
ITIMs
“immunoreceptor tyrosine-based inhibition motif”
sends an inhibitory signal into the cell
ITSMs
“immunoreceptor" tyrosine-based switch motif”
can send either an activating or inhibitory signal into the cell depending on other signals present in the cellular environment
Somatic recombination (DNA rearrangement)
the method by which functional lymphocyte receptor genes are created
involves the rearrangement of gene segments that code for specific portions of lymphocyte receptors into a functional gene
Lymphocyte repertoire
all of the unique TCR and BCR genetic rearrangements within the adaptive immune system
generated by lymphocytes through the recombination of genes
What are the sites of lymphocyte rearrangement and maturation?
rearrangement —> primary lymphoid organ (bone marrow or thymus)
maturation —> secondary lymphoid organ or tissue
Checkpoints for lymphocyte development
After first proliferation, expression of one chain of Pre-B/T antigen receptor —> failure to express results in cell death
After second proliferation, complete expression of immature B/T cell receptor —> strong/absent recognition results in cell death, weak recognition results in maturation
What does single or double positive cells man?
“single positive” cells —> express only one type of surface marker
“double positive” cells —> express both markers simultaneously, indicating an earlier stage of development in the thymus before full maturation
Death by neglect
failure of TCR beta-chain rearrangement during checkpoint 1 —> the cell does not receive a survival signal and eventually dies by apoptosis
Failure of positive selection
failure to interact successfully with self-MHC during checkpoint 2 —> leads to death by apoptosis
Negative selection
too strong of an interaction with any autoreactive cells leads to active initiation of death by apoptosis
Central tolerance
cells that are positively selected, and not deleted by negative selection —> leads to the release of mature single-positive T cells into the circulation
Junctional diversity
the process of DNA sequence variation that occurs when gene segments are joined incorrectly during recombination
this process results in the insertion of additional nucleotides, which can change the amino acid sequence —> contributes to the variability of the CDR3
Combinatorial diversity
contributes to the diversity of B cell receptors
random recombination of separate V, D, and J gene segments to form a complete V-region exon
B-1 B cells
derived from a separate developmental lineage
constitute 30-50% of B cells in pleural and peritoneal cavities of mice
have a relatively limited receptor repertoire
receptors tend to bind microbial carbohydrate anions from microbes generally found in the gut
bind with relatively low affinity
much more similar to PRRs of innate immunity
undergo apoptosis UNLESS they interact with self-antigens
Marginal zone (MZ) B cells
found in white pulp outer regions of the spleen
appear to be specialized for blood-borne Ag recognition
recognize protein and carbohydrate antigens, similar to B-1 cells
some may be able to do so without T cell help
characterized by low levels of IgD and Fc receptors
seem to be derived from T2 cells with strong self-Ag signaling through BCR and binding of Notch ligands
B-2 B cells
mature, primary cells migrate to lymphoid follicles
express high levels of IgM/IgD on their surfaces
recirculate between blood and lymphoid organs
help to respond to antigens with T-cell help by producing antibodies
half-life of approx. 4.5 months in periphery
PD-1
Expression: activated T cells
Function: inhibition of T cell activation
CTLA-4
Expression: regulatory T cells, activated T cells
Function: inhibition of T cell activation
ICOS
Expression: activated T cells, T follicular helper (Tfh) cells
Function: generation of Tfh cells
Central memory T cells (Tcm)
express CCR7 and home to lymph nodes
limited effector function but expand rapidly and gain effector function
Effector memory T cells (Tem)
home to peripheral tissues, especially mucosal tissues
upon stimulation, rapidly produce cytokines like IFN-gamma or become cytotoxic but do not proliferate much
Tissue-resident memory T cells (Trm)
present in non-lymphoid tissues and provide rapid defense against microbes
not in circulation
Peripheral memory T cells (Tpm)
similar to Trm but can enter blood
What is the role of IL-2 in T cell activation?
promotes T cell proliferation, especially for cytotoxic CD8+ T cells
What is the role of the high affinity receptor in T cell activation?
can minimize the number of peptide-ligand interactions required for T cell activation and optimize the signal activation dwell time
Two signals needed to activate naive T cells:
Antigen-specific signal that occurs when the TCR binds to a peptide-MHC complex on an APC
A costimulatory signal that occurs when the co-receptor protein CD28 on the T cell recognizes the B7 proteins (CD80 and CD86) on the APC
What happens if a naive T cell receives signal 1 without signal 2?
it becomes unresponsive or inactivated
What is the role of CD40/CD40L interaction on T cell activation?
leads to dendritic cell expression of B7 —> secretion of cytokines
enhanced T cell proliferation and differentiation
Properties of memory T cells:
express increased levels of anti-apoptotic proteins which may allow them to survive for prolonged periods
respond to antigen more rapidly than naive cells
number of memory cells for a specific antigen are greater than the number of naive cells for that antigen
can migrate into peripheral tissues
undergo a slow proliferation and a long life
survival is dependent on cytokines (IL-7) but does not require antigen
Different types of memory T cells:
Central memory (Tcm)
Effector memory (Tem)
Tissue-resident memory (Trm)
Peripheral memory (Tpm)
How does the immune system respond to microbes that live in the phagosomes of macrophages?
Activates CD4+ effector T cells (T helper cells)
Helps the phagocyte kill the ingested microbe
How does the immune system respond to microbes which do not live in phagosomes?
Activates CD8+ T cells (killer cells)
directly kill the antigens/microbes
What are the CD4+ T cell subsets?
Th1
Th2
Th17
What cytokines are secreted by the CD4+ T cell subsets?
Th1 —> secretes IFN-gamma
Th2 —> secretes IL-4, IL-5, IL-13
Th17 —> secretes IL-17, IL-22
How do leukocytes find sites of infection?
migration of leukocytes to sites of infection is stimulated by cytokines, which induce the expression of adhesion molecules on the surface of endothelial cells and the chemotaxis of leukocytes
What happens to T cells that locate their antigen at a site of infection?
they are activated and retained at the site
What happens to T cells that do not find their cognate antigen at the site of infection?
they return to the circulation, largely through lymphatic vessels
What role does CD40/CD40L and IFN-gamma play in macrophage activation?
IFN-gamma is the major inducer of CD40 expression in macrophages
CD40 is a type of tumor necrosis factor —> expression is crucial for T cell development
CD40L is a costimulatory protein displayed by Th1 effector cells that binds to CD40 on macrophages
How do macrophages facilitate tissue repair?
Granuloma
nodules of inflammatory tissue surrounding particulate sources of antigen
produced when activated macrophages are unable to eradicate an infection
How does the immune system respond to helminth infections?
Using the Th2 differentiation pathway —> chronic T cell stimulation without a significant innate immune response of classical macrophage activation —> activation of eosinophils in response to helminth infection
Two main mechanisms by which CD8+ T cells kill target cells:
Perforin and Granzymes
Fas ligand (FasL)
CD8+ T cell exhaustion
strong and persistent immune responses to chronic antigen exposure can result in damage to host
may have evolved as mechanism to limit immunopathology
repeated antigen stimulation decreases T cell proliferative capacity, and effector function (IFN-gamma production/cytotoxicity)
results in increased expression of inhibitory receptors by T cells
Functions of peripheral gamma-delta T cells
recruited/expand in response to infection
major innate producer of IL-17 —> recruit inflammatory cells (neutrophils) to site of infection
can activate macrophages via production of IFN-gamma or IFN-alpha
possess cytolytic activities (Granzyme B)
Functions of epithelial/mucosal gamma-delta T cells
expand in response to tissue injury
help repair intestinal (colitis) and cutaneous tissue damage
produce tissue growth factor in response to tissue injury
cell-produced growth factors preserve integrity of epithelium under stress
What types of antigens do gamma-delta T cells respond to?
recognize antigen in the absence of MHC
can directly respond to lipid antigens and microbial metabolites during infection —> results in pro-inflammatory cytokine production/macrophage activation to clear infection
can directly respond to molecules released by sterile tissue injury —> results in release of growth factors to repair tissue
T-independent antibody response
in response to multivalent, non protein antigens on microbial surfaces
primarily made by B-1 B cells and marginal zone B cells
they produce low-affinity IgM antibodies and short-lived plasma cells
T-dependent antibody response
in response to protein antigens
primarily made by follicular B cells in response to helper T cells
produce isotype-switched, high-affinity antibodies, memory B cells, and long-lived plasma cells
Why are T-independent responses primarily IgM only?
there is a lack of T-mediated isotype switching processes —> IgM is the first antibody produced in a humoral immune response and does not require isotype switching
Antigen capture and delivery to B cells
in all cases, the antigen captured is presented to B cells generally in its intact, native conformation, and is not processed by antigen-presenting cells
What second signals can fully activate a B cell?
signal provided by complement receptors (CR2), pattern recognition receptors (PRRs), or T cell help is usually required for full activation
How do T cells and B cells move toward each other in the lymph node?
Migration of activation T cells to edge of follicle —> upregulation of CCR7 and downregulation of CXCR5
B cells present antigen to activated helper T cells —> B cells interact with T cells that recognize the same antigen
Antigen uptake and processing —> B cell activation
migration of activated B cells to edge of follicle —> expression of EBI2 and upregulation of CCR7
What is the role of CD40-CD40L in B cell activation?
activated helper T cell expresses CD40L, secretes cytokines
B cells are activated by CD40 engagement, produce cytokines
B cell proliferation and differentiation
Six broad categories of antibody effector functions:
Neutralization —> protects against viral or bacterial infection or the effects of toxins
Agglutination —> enhances neutralization and more efficient clearance of pathogens from the body
Opsonization —> promotes and/or enhances the engulfment of antigens by phagocytes
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) —> activates the killing activity of several types of cytotoxic cells
Antibody-Dependent Degranulation and Mediator Release —> triggers mediator release from granulocytes
What is cross-presentation?
antigens presented on class I MHC molecules come from the cytosol and are processed through the endogenous pathway
microbes that do not infect dendritic cells will be acquired from outside the DC; the same will occur for tumor antigens or cells
in order to be presented on class I MHC molecules, classical DC1 subset cells are used to ingest infected cells, tumor cells, or proteins produced by these cells, transfer these proteins into the cytosol, and process them for class I presentation
Perforin
exocytosed in CTL granules and polymerizes in the target cell plasma membrane, forming pores
induces uptake of granzymes into target cell endosome and release into cytosol, activating caspases
Granzymes
exocytosed in CTL granules, enter target cells through the perforin pores, and induce target cell apoptosis
Fas ligand (FasL)
expressed on activated CTLs, engages Fas (“death” receptors) on the surface of target cells, and induces apoptosis
Primary humoral response
occurs immediately after antigen exposure
smaller in magnitude
antibody isotype is usually IgM > IgG
lower average antibody affinity; more variable
induced by all antigens
Secondary humoral response
occurs a few days after antigen exposure
larger in magnitude
antibody isotypes: relative increase in IgG, often IgA, and sometimes IgE
higher average antibody affinity (affinity maturation)
induced by protein antigens only
Effector Functions of Antibodies
Neutralization of microbes and toxins
Opsonization and phagocytosis of microbes
Antibody-dependent cellular cytotoxicity
Phagocytosis of microbes opsonized with complement fragments
Inflammation
Lysis of microbes
Complement activation