1A
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
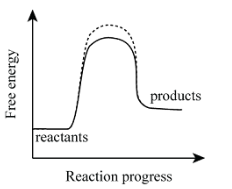
Is this an endergonic or exergonic reaction?
Endergonic
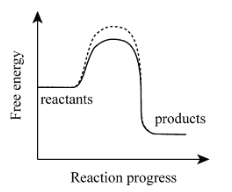
Is this an endergonic or exergonic reaction?
Exergonic
Would the formation of solvation layers increase or decrease entropy? Why?
Decreases entropy because it maximizes the organization of water molecules through hydrogen bonds
In electrolytic cells the anode is ____ charged. If a an amino acid moves toward the anode it is ____charged. The point in which it can’t move is it’s _______. We can try to stop the amino acid by making it more _____ thus decreasing the _____.
Positively, negatively, isoelectric point, positive, isoelectric point
What are secretory proteins?Where do they belong?
Secretory proteins are proteins that are exported from the cell or delivered to the cell membrane, lysosomes, or other organelles.
Synthesized by ribosomes on the RER
Folded and modified in the RER
Packaged in vesicles and sent to the Golgi apparatus
Further modified and sorted
Transported in secretory vesicles to:
The plasma membrane (for exocytosis)
Lysosomes
Or embedded into membranes
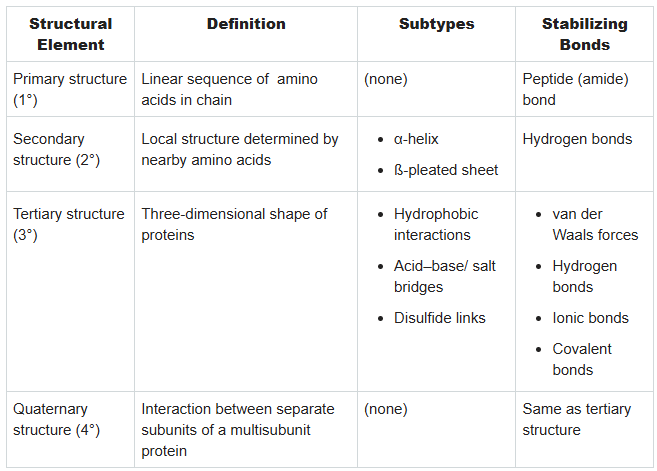
What are the definitions, subtypes, and stabilizing bonds for each part of protein?
Primary:
Secondary:
Tertiary:
Quaternary:
What is a conjugated protein and what are two types?
Proteins with prosthetic group like glycoproteins and lipoproteins
Is quaternary structure absolutely necessary for enzymatic activity?
No
What do suicide inhibitors do?
It irreversibly inhibits enzymes
Km symbolizes the ___, which is not altered by increasing the _____.
Affinity of enzyme to substrate (High Km =Low affinity) enzyme concentration.
How do we know which is the rate limiting step based on the Km?
When it has a high Km, that means we have low enzyme-substrate affinity and therefore a slow reaction velocity.
How do you calculate the pI for AA with the following side chains
Neutral:
Basic:
Acidic:
Neutral: Average pKa values for the main chain carboxyl group and main chain amino group
Basic: Average pKa values for the main chain amino group and the side chain
Acidic: Average pKa values for the for the main chain carboxyl group and the side chain
If there is a certain subunit of a protein that mentions of some sort of reaction happening at something like P402, what does that mean?
The reaction is happening at proline at position 402
what is a potential benefit of glycine?
Because of how small it is , it allows more protein interactions like turns!
If we remove a cofactor from its enzyme, what would happen?
activity IMMEDIATELY drops off
When we add an ATP to a reaction it serves primarily to ___ and not to___.
Activate another reactant
phosphorylate
What is a tripeptide? If we have 3 tripeptides and none can have the same amino acid sequence how many toal structures can we make?
For every tripeptide there are 3 AAs that can only be arranged 2 ways so there can only be 6 structures.
If we add a negative species to something what happens to its isoelectric point?
If we want a net charge of 0, the pI =pH, so if we are more negative we have a lower pH and we will therefore need a lower pI.