Important Steps, Reactants, and Products of Cellular Respiration
1/49
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
50 Terms
Four Stages of Cellular Respiration and Where They Occur
Glycolysis - 10 step process within cytoplasm
Pyruvate Oxidization - 1 step process within mitochondrial matrix
Kreb’s Cycle - 8-step cyclic process within in the
mitochondrial matrix
Electron Transport Chain - Multistep process in the inner
mitochondrial membrane
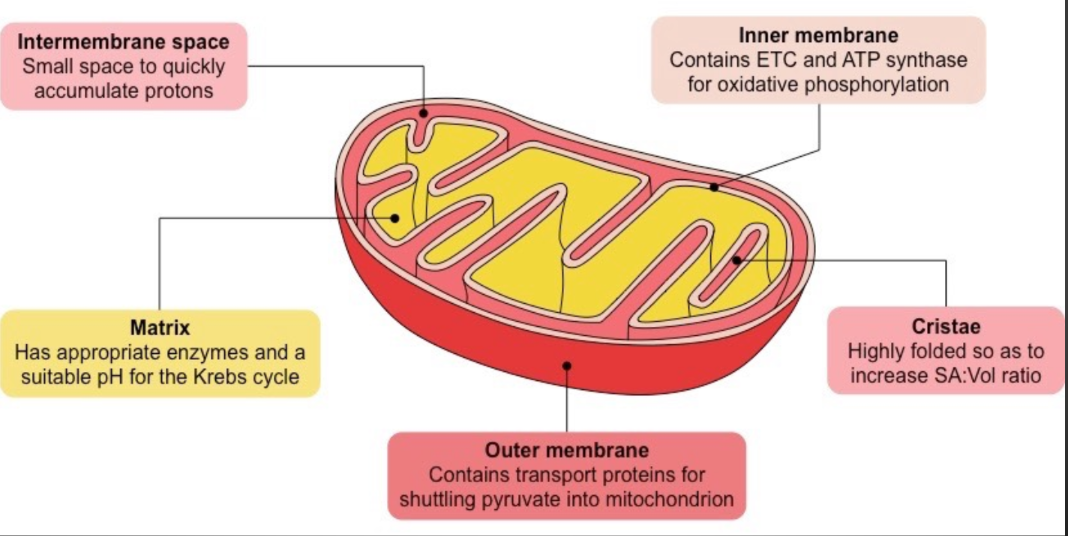
Mitochondrion
Membrane bound organelle
Referred to as a powerhouse because it generates most of the ATP with the Kreb’s cycle and Electron Transport Chain
Composed of an inner and outer membrane
Intermembrane space - is space between inner and outer membrane
Matrix - is the interior aqueous environment inside the inner membrane
What is the goal of cellular respiration? How is this accomplished? (Hint: 2 mechanisms to produce ATP)
To capture free energy in the form of ATP
Substrate-Level Phosphorylation
Oxidative Phosphorylation
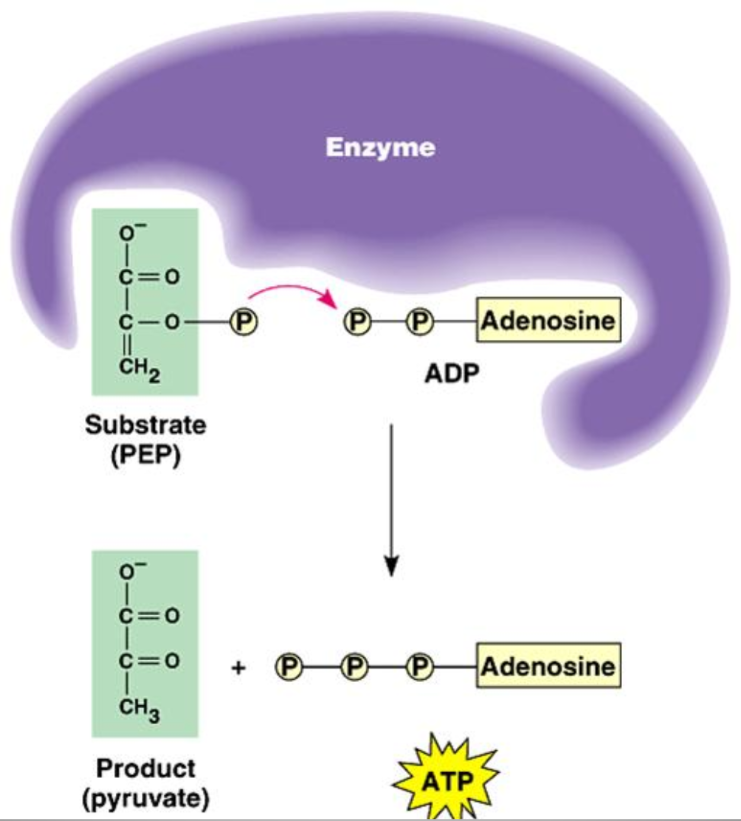
Substrate-Level Phosphorylation
Process in which ATP is formed directly, in an enzyme-catalyzed reaction:
A phosphate group is transferred from a molecule to an ADP molecule
Eg. A phosphate containing molecule called phosphoenolpyruvate (PEP) transfers its phosphate group to ADP, forming ATP
Occurs in Glycolysis (step seven & ten ) and The Citric Acid Cycle (Kreb’s Cycle on step 5)
Oxidative Phosphorylation
ATP formed indirectly through a series of enzyme-catalyzed redox reactions involving oxygen as the final electron acceptor
REDOX Reactions: A pair of reactions where one molecule gains an electron to become REDUCED and another molecule loses an electron to become OXIDIZED
“LEO the Lion says GER”
What yields more ATP; O.P or S.L.P? Where does O.P occur? What two enzymes does it involve?
Yields more ATP than substrate-level phosphorylation
*Occurs in Glycolysis, ETC
Uses two coenzymes:
Nicotinamide Adenine Dinucleotide (NAD+)
Flavin Adenine Dinucleotide (FAD)
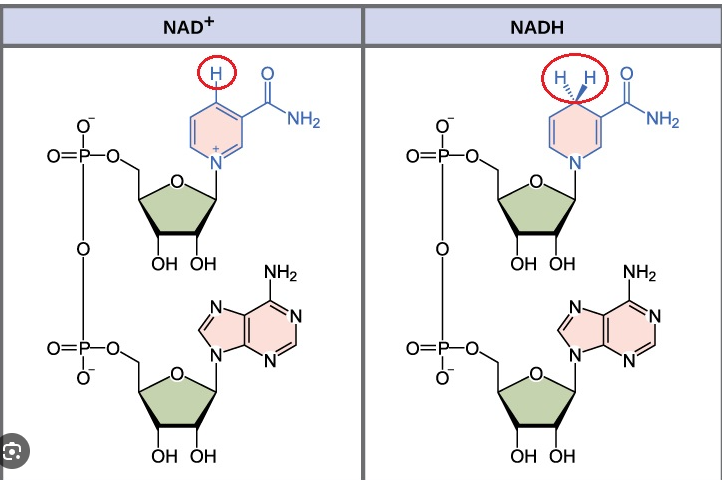
How many hydrogen atoms does NAD+ remove from the original glucose molecule? How many electrons attach to NAD+, reducing it to NADH?
NAD+ is an electron carrier (coenzyme)
NAD+ removes two hydrogen atoms (two protons and two electrons) from original glucose molecule
Two electrons and one proton attach to NAD+, reducing it to NADH
Remaining proton dissolves in solution as H+
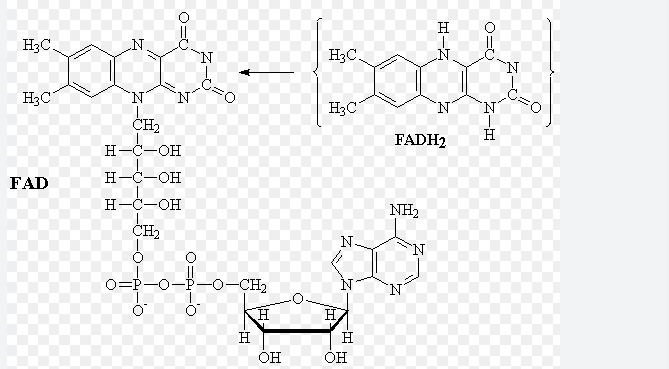
How is FAD reduced? Are any protons released when they bind to FAD? When does it occur?
Also reduced by two hydrogen atoms of the original glucose molecule.
Its’ reduced form is FADH2
All protons and electrons of hydrogen bind directly to FAD
Occurs as one-step reaction of the Kreb’s Cycle
Aerobic Cellular Respiration
Process that extracts energy from food in the presence of oxygen
Energy used to make ATP from ADP and inorganic Pi
ATP used to supply energy directly to cells
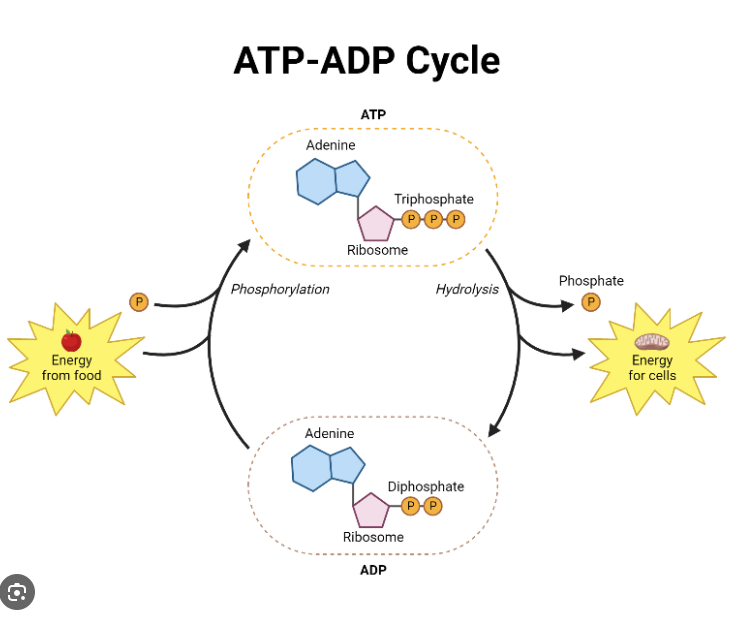
ATP: Adenosine Triphosphate (ATP-ADP Cycle) What reaction occurs when cells need energy?
Primary source of Free Energy in living cells
Free energy is energy that can do useful work
Contains nitrogenous base: adenine, ribose sugar and a chain of 3 phosphate groups
When cells need Free Energy: ATP undergoes hydrolysis (reaction with water), breaking the bond between the second and third phosphate groups.
Converts ATP to ADP (ATP → ADP + Pi + Energy)
After the energy is used, ADP can be converted back to ATP through a process called phosphorylation, which adds a phosphate group back to ADP
ADP + Pi + Energy → ATP
This releases 31 kJ/mol of Free Energy
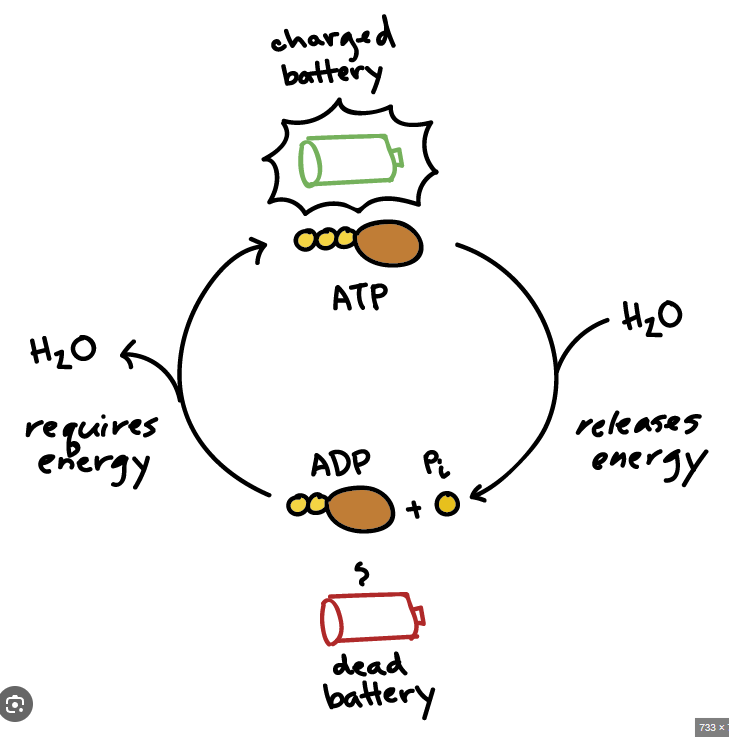
Think of a rechargable battery
Regenerating ATP
Cells generate ATP by combining ADP with Pi (Phospahte)
This is called a phosphorylation reaction
ATP synthesis requires the input of energy (endergonic process)
Energy needed to make ATP comes from breakdown of complex molecules which contain an abundance of energy (exergonic process)
These complex molecules are carbohydrates proteins and fats
Cellular Respiration
The breakdown of glucose to make energy (ATP) using oxygen
Overall equation:
C6H1206 +602 → 6CO2 + 6H20
Ultimate Goal: To extract energy from nutrients and store it as ATP
Achieved by:
Breaking bonds between the 6 carbon atoms in glucose, creating 6CO2’s
Moving hydrogen atoms from glucose to oxygen, for 6H20’s
Glycolysis (Beginning of Process) + What does the name literally mean?
First 10 reactions of cellular respiration
Greek for “sugar splitting”
Starts with glucose (6C sugar) and produces two 3C pyruvate (pyruvic acid) molecules
Occurs in cytoplasm
Anaerobic process: Does not require oxygen
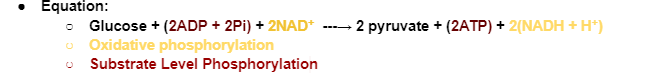
Glycolysis Reactants
1 Glucose Molecule (C6H12O6, 6-C)
2 ATP Molecules (1-5 Energy Investment Phase, Step 1 & 3)
2 NAD+ (Electron Carriers)
Glycolysis Products
2 Pyruvate Molecules (3-carbon molecules)
4 ATP Molecules (Net gain: 2 ATP, since 2 were used, 6-10 Energy Yielding Phase)
Two ATP molecules are invested during the energy investment phase to help prepare glucose for further breakdown (destabilize glucose, prepare it for splitting)
2 NADH molecules (Electron Carries)
Glycolysis Analysis
The overall goal of glycolysis is to break down glucose into smaller pyruvate molecules, producing a small amount of ATP and NADH for energy use.
Free Energy (Does it satisfy the energy requirements of multicellular organisms?)
Glycolysis transfers only 2.2% of free energy available in 1 mol of glucose to ATP
Not efficient at harnessing energy -does not satisfy the energy needed of most multicellular organisms
Thought to be earliest form of energy metabolism used by simplest anaerobic organisms
Most energy still stored in two pyruvate and two NADH molecules
Pyruvate Oxidation
Following glycolysis, two pyruvate molecules are transported through the two mitochondrial membranes into the matrix
The main purpose of pyruvate oxidation is to convert pyruvate (from glycolysis) into acetyl-CoA, which can then enter the Krebs cycle (Citric Acid Cycle) for further energy extraction.
This process also generates NADH and releases carbon dioxide (CO₂).
Reactants in Pyruvate Oxidation
2 Pyruvate Molecules (From glycolysis)
2 NAD+
2 Coenzyme A (COA)
Steps of Pyruvate Oxidation
A multi-enzyme complex catalyzes 3 changes before Krebs cycle:
#1: Decarboxylation Reaction
Low energy carboxyl group (COOH) is removed as CO2
#2: Redox Reaction
Remaining 2-C portion is oxidized (loses electrons) by NAD+ and forms an acetyl group (acetate)
NAD+ reduced (gaining electrons) to NADH (plus) H+ (oxidative phosphorylation)
#3 Formation of Acetyl-CoA
Sulfur-containing compound called coenzyme A (CoA) is attracted to the acetate component, forming acetyl-CoA
Overall Goal: To change pyruvate into acetyl-CoA
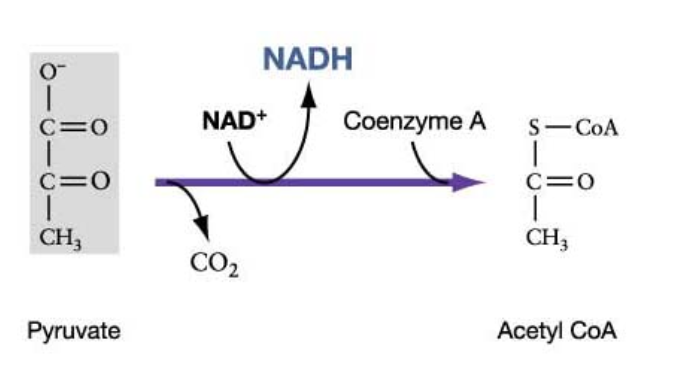
Products of Pyruvate Oxidation (Per Glucose Molecule)
2 Acetyl - Coa (One from each pyruvate)
2 NADH
2 CO2
Where do Products of Pyruvate Oxidation Go?
2 molecules of Acetyl-CoA enter the Krebs cycle, where more free energy transfers occur
2 molecules of NADH proceed to ETC and Chemiosmosis to produce ATP by oxidative phosphorylation
2 CO2 molecules diffuse out as waste (exhalation)
2 H+ stay dissolved in mitochondrial matrix

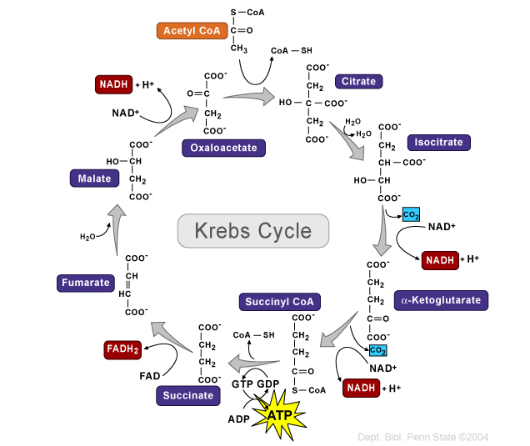
The Citric Acid Cycle
A.K.A Kreb’s Cycle
8 enzymes catalyzed reactions
Result in oxidation of acetyl group to CO2
Completes the conversion of all carbon atoms originally in glucose to CO2
Synthesizes ATP, NADH and FADH2
Its main purpose is to fully oxidize the acetyl group from acetyl-CoA into carbon dioxide (CO₂) and capture high-energy electrons in the form of NADH and FADH₂ for use in the electron transport chain.
The cycle also generates a small amount of ATP (or GTP).
Reactants (Per cycle)
1 Acetyl-CoA (2-carbon molecule from pyruvate oxidation)
3 NAD⁺
1 FAD
1 ADP (or GDP) + Pi
Products
2 CO₂ (carbon dioxide)
3 NADH
1 FADH₂
3 H+
1 ATP (produced via substrate-level phosphorylation) (Step 5)
CoA (regenerated)
For one glucose molecule (since glucose generates 2 acetyl-CoA molecules)
4 CO₂
6 NADH
2 FADH₂
2 ATP (Step 5)
Kreb’s Cycle Summary
The Krebs cycle happens twice per glucose molecule because glycolysis splits one glucose into two pyruvate molecules, which are converted to two acetyl-CoA molecules.
The cycle is essential for generating high-energy electron carriers (NADH and FADH₂), which provide electrons to the electron transport chain for ATP production via oxidative phosphorylation.
Overall, the Krebs cycle produces the reducing agents (NADH and FADH₂) needed for the final and most significant ATP-producing step of cellular respiration.
The Krebs cycle begins and ends with a molecule called oxaloacetate. It combines with a 2-carbon molecule (acetyl-CoA) to start the cycle, and after a series of reactions, it gets regenerated back to oxaloacetate so the cycle can start over again.
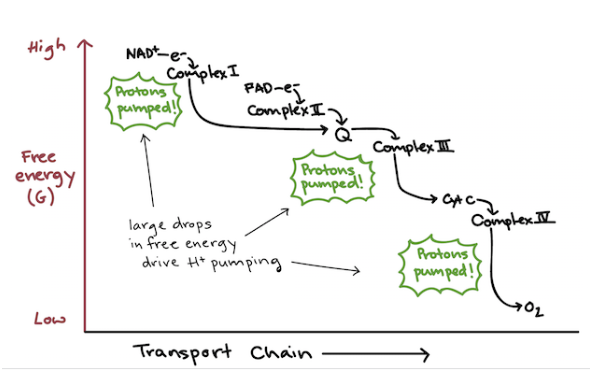
Electron Transport Chain
Facilitates transfer of electrons from NADH and FADH2 to O2, ultimately producing ATP
Series of four protein complexes built into inner mitochondrial membrane:
Flow of electrons facilitated by two electron shuttles:
1 UQ: Ubiquinone
Hydrophobic, found in the core of the membrane
Shuttles electrons from complex I and II to complex III
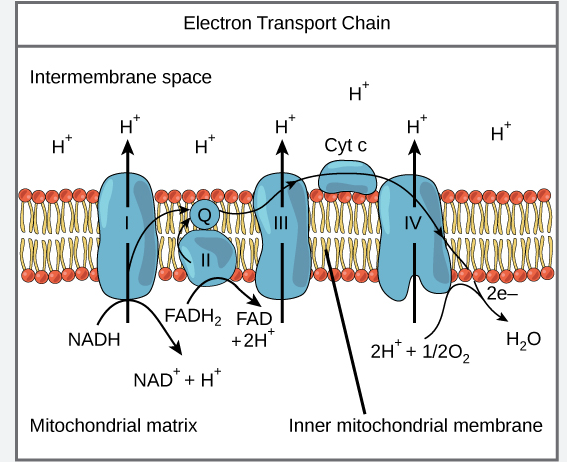
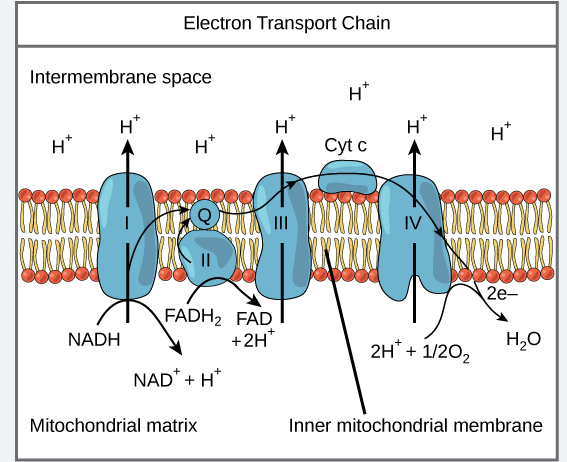
2 Cyt: Cytochrome c
On intermembrane space side of membrane
Transfers electrons from complex III to IV
Electron Transport Chain Continued
Each complex lies in order of increasing electronegativity
Each compound is alternately reduced (gain e-) and oxidized (lose e-)
Controlled release of energy
When e- reaches last protein, it becomes very stable
Chain starts of in NADH and FADH2, then pass electron to ETC
NADH and FADH2 are oxidized
H’s are removed and electrons are stripped from hydrogen atoms and become hydrogen ions
2 electrons are passed from one electron (NADH Or FADH2 → O2) carrier to the next
Removed from complex IV by oxygen (since oxygen has a higher electronegativity than complex IV and therefore has the ability to pull electrons without backing the ETC)
Oxygen
One of the most electronegative elements
Oxidizes complex IV by stripping last electrons from it
Oxygen then joins with 2 protons (hydrogen ions) and form water
½ O2 + 2e- + 2Hᐩ → H2O
Free Energy
Free energy lost by electrons at each step/complex (exergonic) is used to pump hydrogen ions from mitochondrial matrix into intermembrane space
This sets up a hydrogen ion concentration and electric gradient (electrochemical gradient) across the membrane, which is used to phosphorylate ADP → ATP
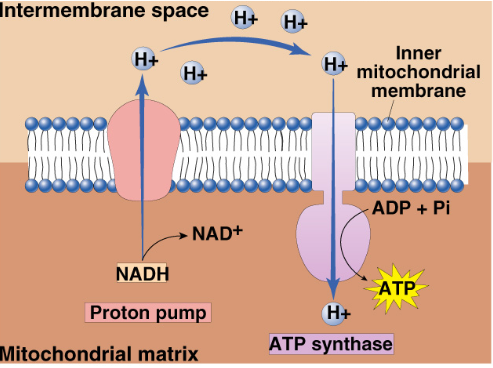
NADH
Passes electrons to NADH dehydrogenase (1st protein complex)
NADH oxidation pumps 3 protons and are responsible for producing 3ATP
FADH2
Passes electrons to complex II
FADH2 oxidation pumps 2 protons and are responsible for producing 2ATP
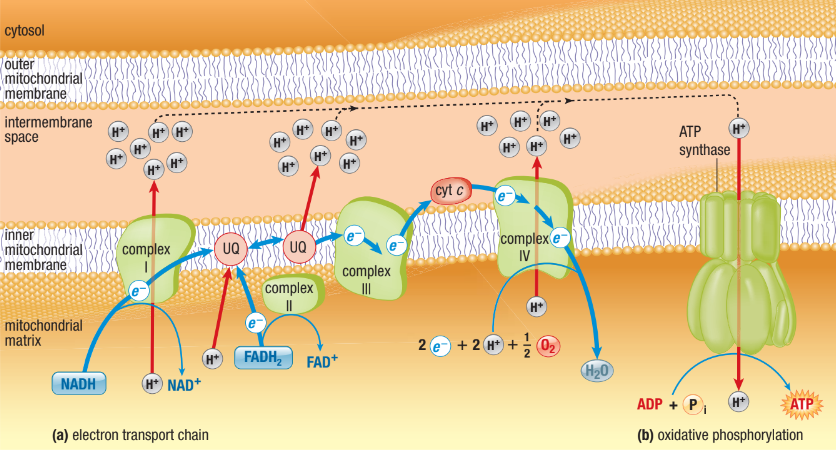
Chemiosmosis + ATP Synthase
Enzyme spans inner membrane of mitochondria
ADP + P1 → ATP
Only channel permeable to hydrogen ions
Hydrogen ions flow down concentration gradient back into the matrix and so provides energy for ATP synthesis
Flowing hydrogen ions causes change in the shape of ATP synthase enzyme
Powers bonding of P1 to ADP
Called “protein-motive” force
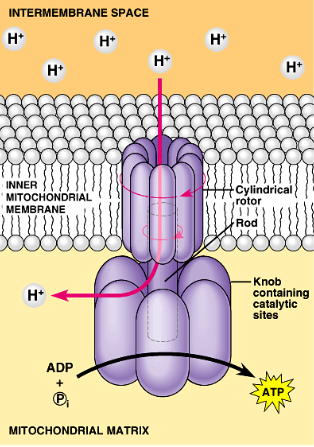
ATP Synthase Con.
Chemiosmosis couples ETC to ATP synthesis
Use of hydrogen gradient to transfer energy from redox reactions to cellular work (ATP synthesis)
The Problem with Glycolysis
Glycolysis produces 2 NADH molecules in the cytoplasm
These NADH molecules cannot add their electrons to the ETC because they are not in the mitochondria
The electrons in NADH can use one of two different shuttle systems to add their electrons into the ETC.
The two shuttle systems
Malate - Aspartate Shuttle
Glycerol - Phosphate Shuttle
Malate-Aspartate Shuttle
NADH in cytosol is oxidized to NAD+
An organic compound is reduced to Malate
The electrons are transferred into the mitochondrial matrix in malate
They reduce another NAD+, forming NADH in the matrix
NADH then adds the electrons to the ETC as normal
The NADH in the cytosol is oxidized to NAD*, and the electrons are transferred across the membrane and used to reduce an NAD+ to NADH within the matrix.
Glycerol-Phosphate Shuttle
NADH in cytosol is oxidized to NAD+
Glycerol phosphate is reduced
The electrons are transferred into the mitochondrial matrix in glycerol phosphate
Glycerol phosphate reduces an FAD, forming FADH2 in the matrix
FADH2 then adds the electrons in the ETC as normal
Little less efficient than the malate-aspartate shuttle
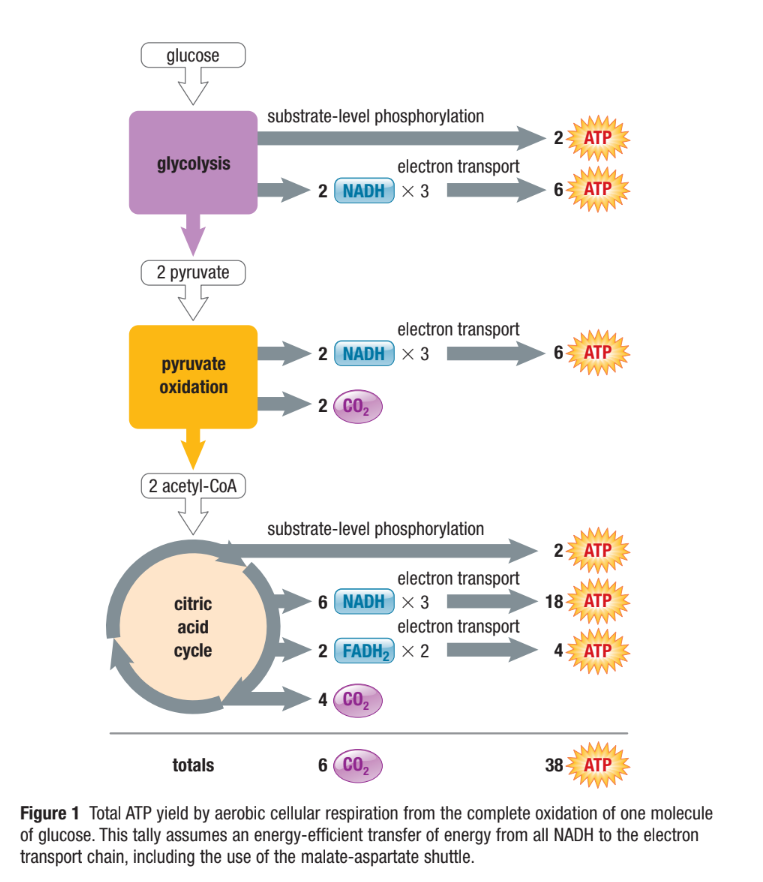
Efficiency of ATP Production
Many reasons why total yield may be less than max 38 ATP
Eg. Energy from H+ flow is lost due to other mitochondrial processes or using the glycerol phosphate shuttle
Only about 42% of total energy in glucose is converted to ATP
Rest is lost as thermal energy
Cars only convert 25% of the energy from fuel into motion
Fermentation
Two anaerobic pathways that do NOT use oxygen as the final electron acceptor
Glycolysis is the first step of the fermentation pathways
Final steps only serve to regenerate NAD+ → the coenzyme needed to run gylcolysis
Alcohol Fermentation
Yeast (fungus) used in beer brewing and wine making, as well as some bacteria
2 molecules of pyruvate are formed, along with 2 ATP and 2 NADH
Each pyruvate formed from glycolysis rearranged into acetalehyde through a decarboxylation reaction (intermediate)
Acetaldehyde accepts hydrogen and electrons from NADH
Then converted into ethanol (As a result of being reduced)
Glucose → 2 ethanol + 2CO2 + 2ATP
Lactate (Lactic Acid Fermentation)
Fungi, bacteria used in dairy industry to make cheese and yoghurt
Eg. Lactobacilli and streptococci ferment lactose in milk to lactic acid
Begins with glycolysis (2 pyruvate, 2 ATP, 2 NADH)
Pyruvate is converted into lactic acid (Reduced)
Human muscle cells make ATP by lactic acid fermentation when oxygen is scarce (during strenuous exercise)
Increase lactate causes fatigue and pain (harmful to muscles)
Lactate is gradually taken by blood to liver
Liver converts lactate back to pyruvate
Glucose → 2 lactic acid + 2 ATP
Fermentation vs. Respiration: How are they similar?
Both use glycolysis to oxidize glucose into pyruvate
Both produce net yield of 2ATP (substrate level phosphorylation)
Use NAD+ as oxidizing agent: accepts electrons from food during glycolysis
Fermentation vs. Respiration: How are they different?
#1 NADH is oxidized back to NAD+
Fermentation
NADH passes electrons to pyruvates or some derivative (acetaldehyde)
Electrons are NOT used to power ATP production
NADH oxidizes because its electrons are passed to pyruvate (acetaldehyde)
Respiration
Electron transport (ETC) from NADH to oxygen drives oxidative phosphorylation and regenerates NAD+
Electron Transport Chain oxidizes NADH
#2 Final Electron Acceptor
Fermentation:
Pyruvate (latic acid fermentation)
Acetaldehyde (alcohol fermentation)
Respiration:
Oxygen
#3 Amount of Energy Harvested
Fermentation:
Energy stored in pyruvate is unavailable to cell
Respiration:
18 times more ATP
Krebs cycle completes oxidation of glucose
Taps into energy stored in pyruvate at end of glycolysis
#4 Requirements of Oxygen
Fermentation:
No need for O2
Respiration:
Only occurs in the presence of oxygen