Electrolysis of Aqueous solutions 2
1/3
Earn XP
Description and Tags
https://www.youtube.com/watch?v=mL7mkqyLpSo&list=PL9IouNCPbCxXDlRtCQEG0cGehBvJ7t9Pf&index=17
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
4 Terms
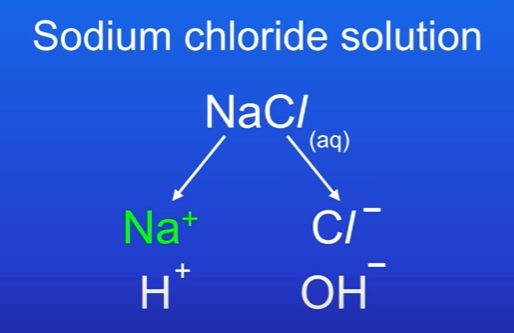
Explain electrolysis of sodium chloride
Has Na+ and Cl-, also H+ and OH- from water.
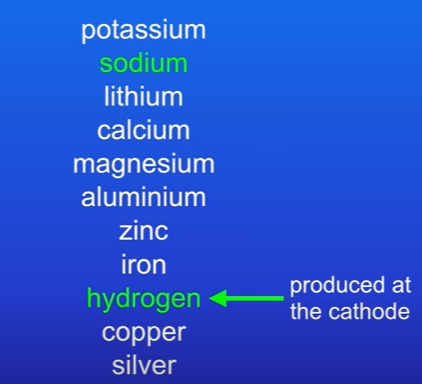
The Na+ and H+ will be attracted to cathode. Hydrogen is produced at cathode if metal is more reactive than hydrogen. By looking at reactivity series, it shows that calcium is more reactive than hydrogen , therefore hydrogen will be produced at the cathode.
At the anode, Cl- and OH- will be attracted. If the aqueous solution contains halide ions, then the halogens will be produced at the anode, therefore at the anode we make chlorine gas.
Which 2 ions will be attracted to cathode ( neg electrode ) during electrolysis of sodiium chloride
H+ and Na+
Whats key fact about anode
If the aqueous solution contains halide ions, then the halogens will be produced at the anode.
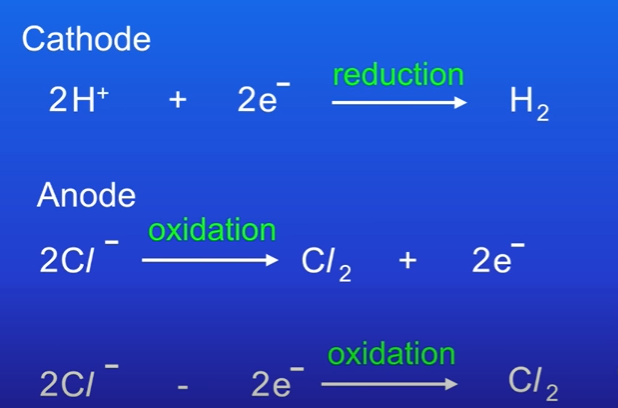
Explain half reactions of sodium chloride.
Hydrogen atoms immediately pair to produce H2, so equation must be doubled.
Chlorine atoms always pair to form chlorine molecule, so all must be doubled.