C4: Electrochemistry
1/6
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
7 Terms
Key terms:
Cation
Anion
Cathode
Anode
Electrolyte
Cation: Positive ions
Anion: Negative ions
Cathode: Negative electrode
Anode: Positive electrode
Electrolyte: A liquid or solution that can conduct electricity
What does it means by Electrolysis?
Electrolysis means splitting up with electricity.
What is the definition of Electrolysis?
Electrolysis is the breakdown of an ionic compound (molten or in aqueous solution) by the passage of electricity.
Which ions will form at cathode and anode and why?
Cation (positive ion) will form at cathode
Anion (negative ion) will form at anode
The reason is because positive ion will be attracted to the negative electrode, and the negative ion will be attracted to the positive electrode.
What products will be formed at cathode and anode when using molten electrolyte?
At the cathode: Metal form
At the anode: Non-metal form
What products will be formed at cathode and anode when using dissolved eletrolyte (aqueous solution)?
At the Anode (+):
If the electrolyte is a concentrated solution of halide, a halogen forms.
Otherwise, Oxygen forms
At the Cathode (-):
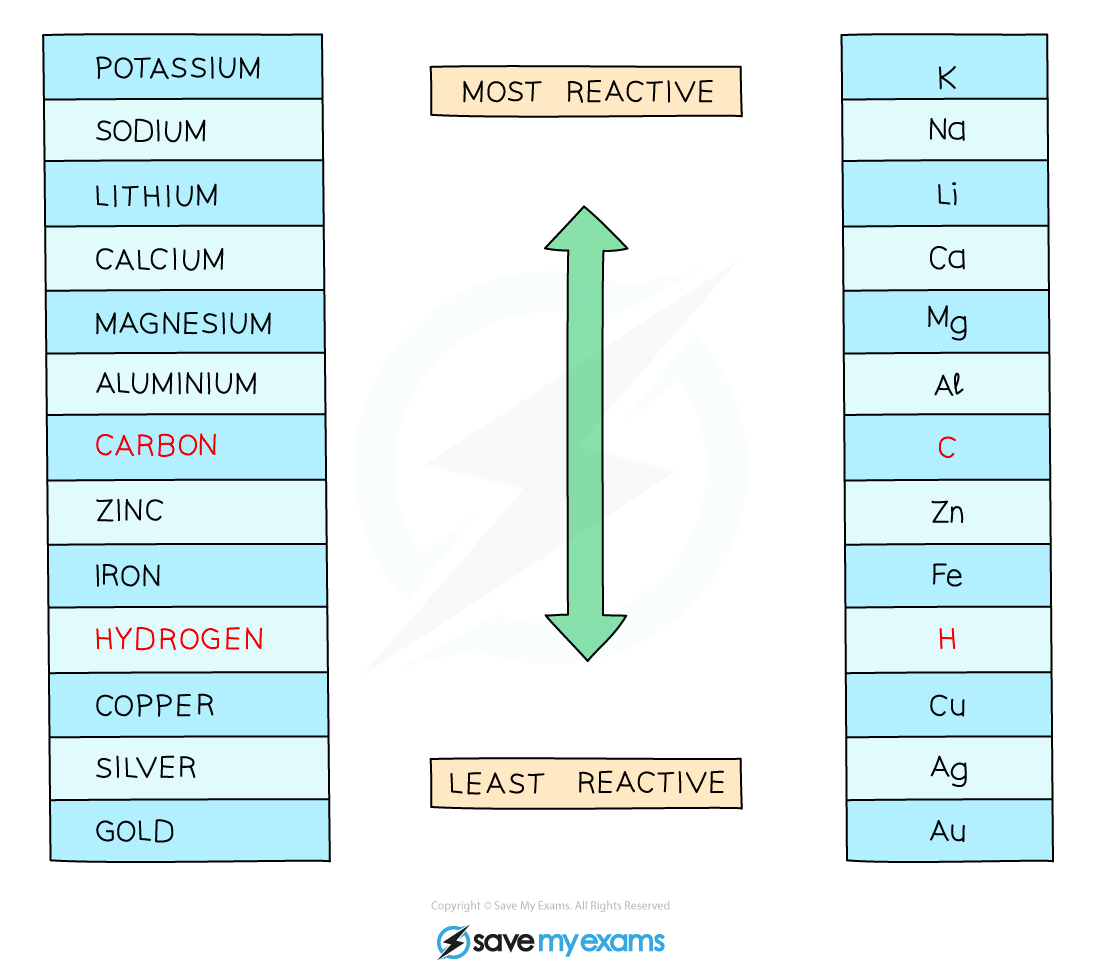
If the metal is less reactive than Hydrogen, then The Metal forms.
Otherwise, Hydrogen forms