Unit 6- Amines & Amides
1/6
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
What is an amine?
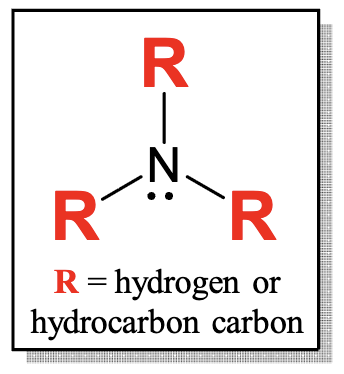
nitrogen in between a combination of hydrogen atoms and hydrocarbon-type carbons
nitrogen=amino group
weakly basic because of very active lone electron pair on N
Classification of amines & amides
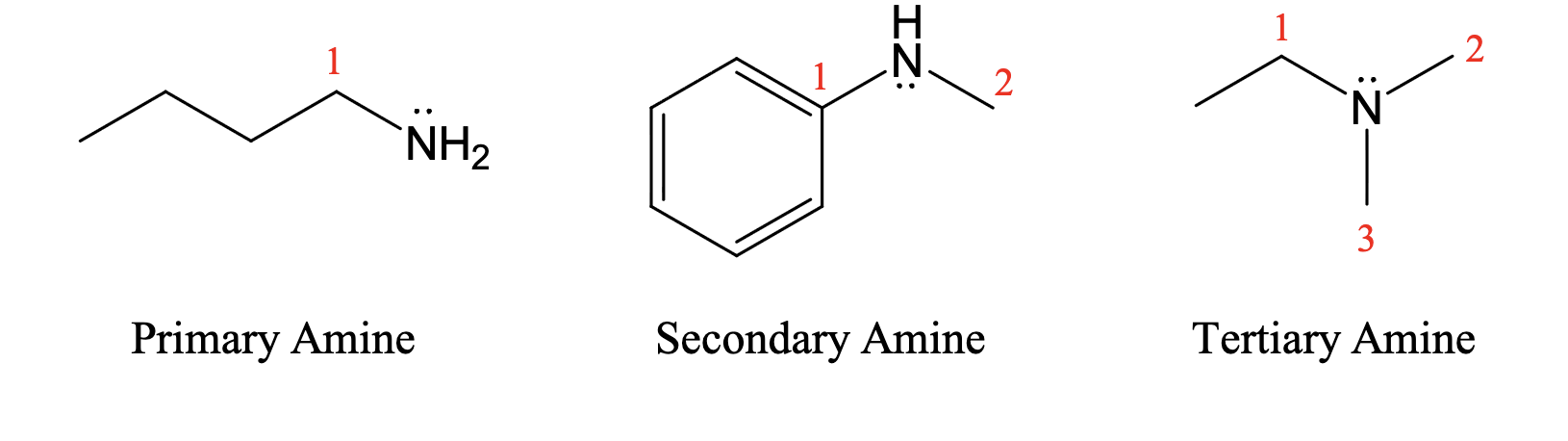
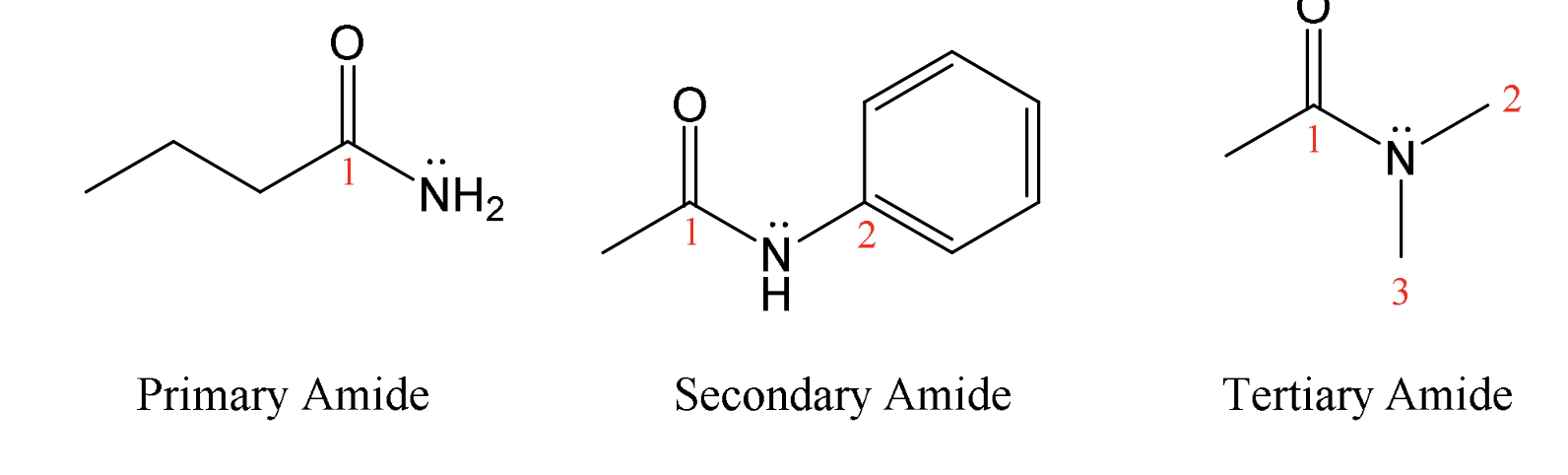
(tertiary amines cannot hydrogen bond therefor lower bp)
IUPAC Nomenclature for amines
find longest continuous carbon chain containing amino group (N)
remove “-e” from corresponding alkane name and replace it with “-amine”
number parent chain giving amino group lowest numerical designation
name secondary and tertiary with “N-alkyl” prefix
Acid-Base reaction of amines
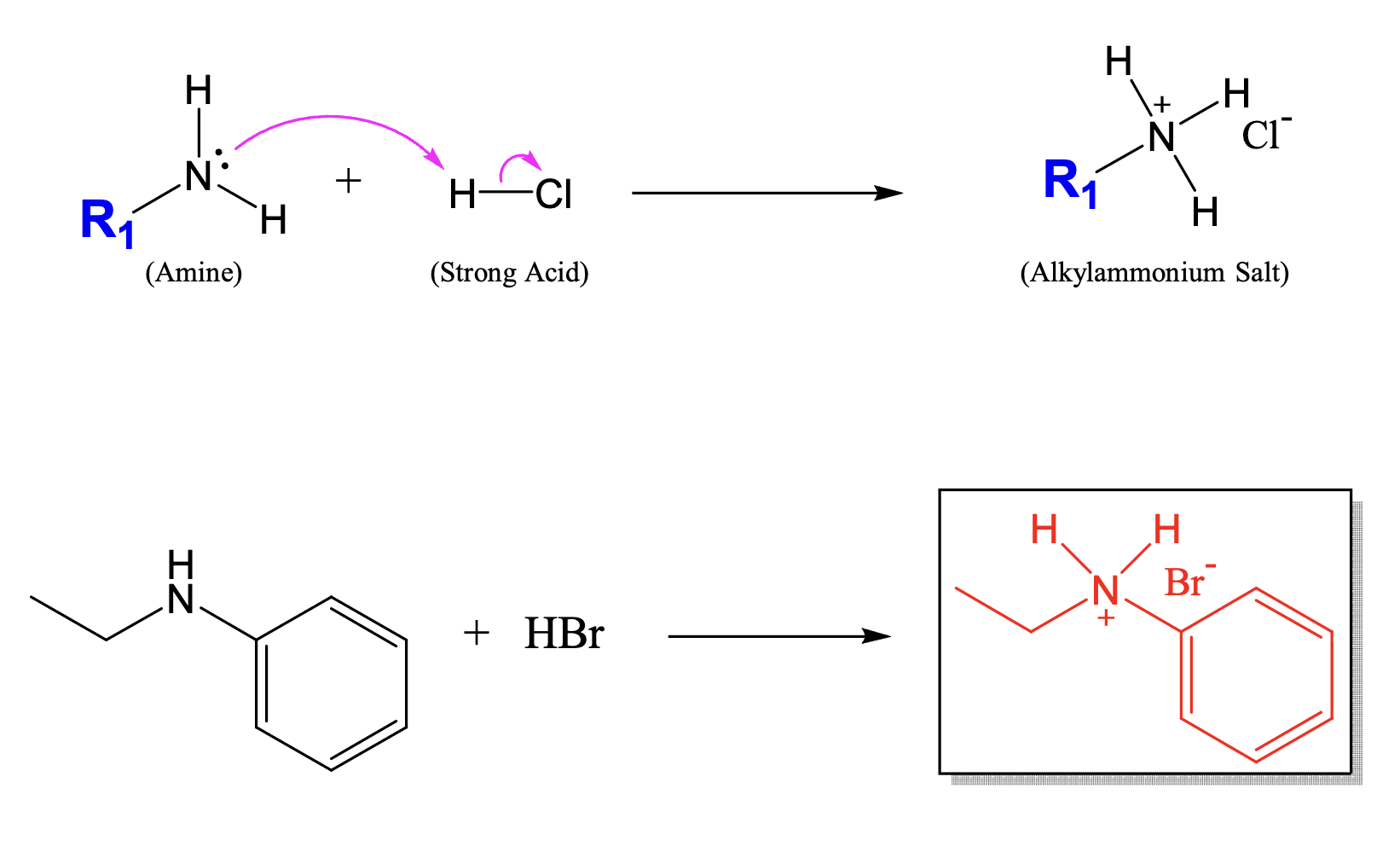
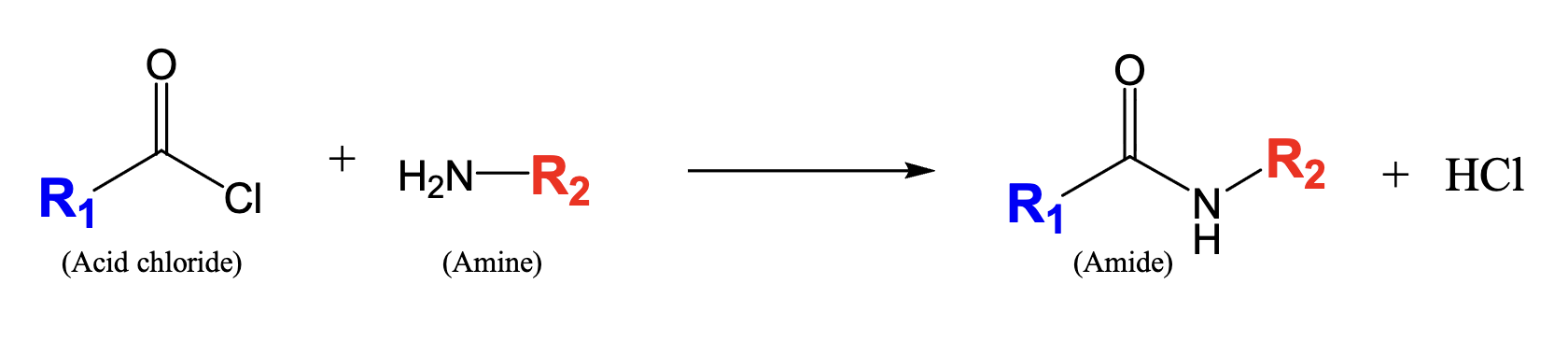
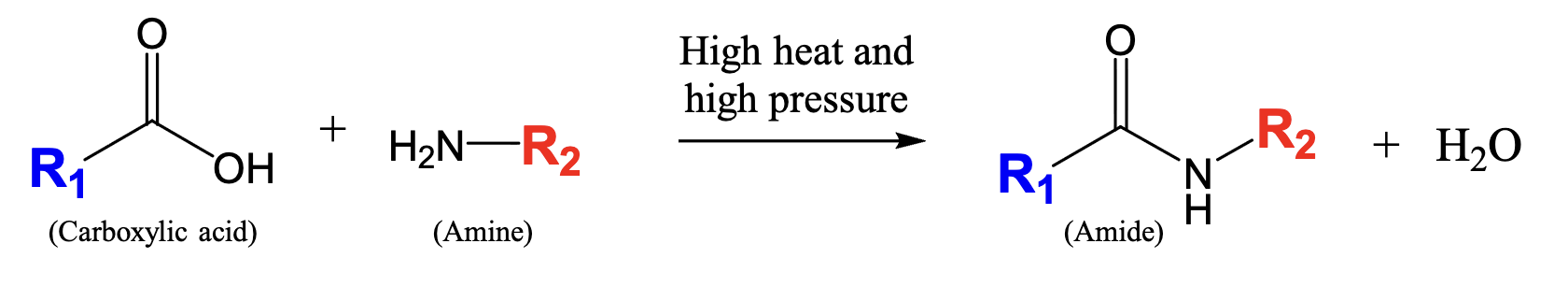
What is an amide?
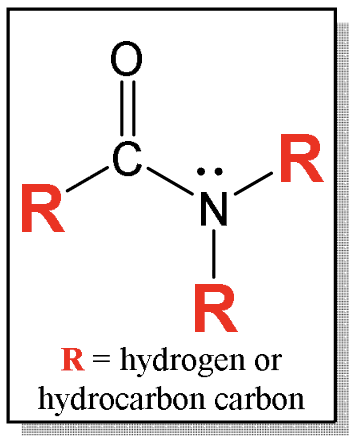
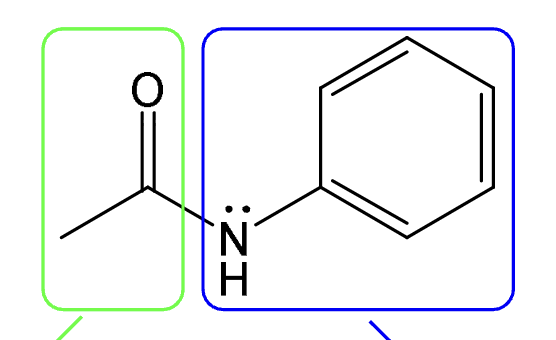
Nitrogen between carbonyl carbon (C==O) and combination of hydrogens or hydrocarbon-type carbons
fusion between carboxylic acid and amine functional group
not basic like amines
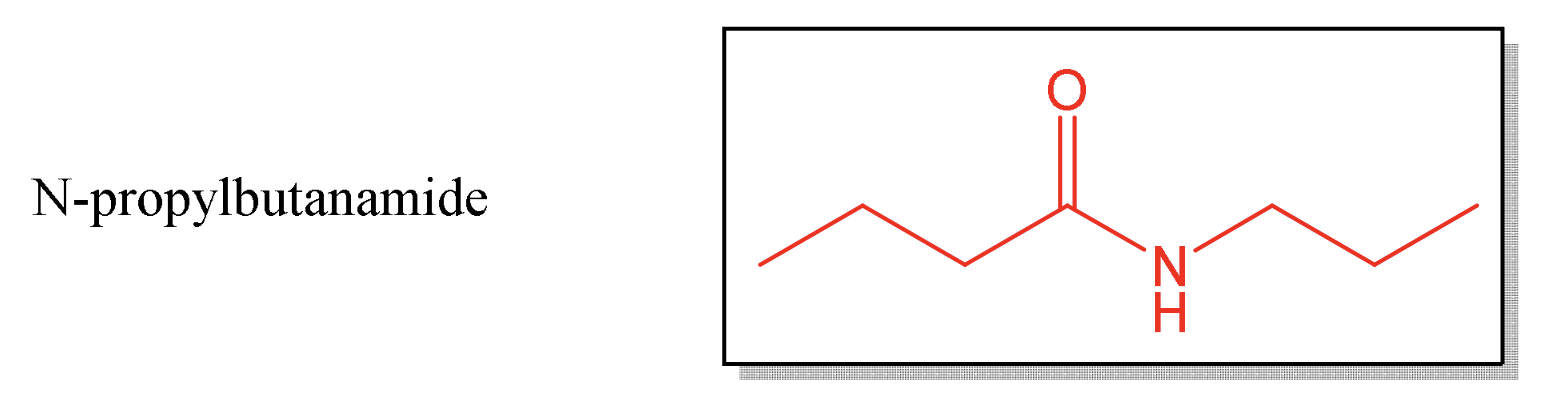
IUPAC nomenclature for amides
find longest continuous chain of carbons containing amide carbonyl
remove “-e” from corresponding alkane name replacing it with “-amide”
no need to number parent chain because 1 designation always on carbonyl carbon
name secondary and tertiary amides with “N-alkyl” prefix
Amide reactions