Bioc 203: PDC and Krebs Cycle
1/22
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
23 Terms
PDC
Pyruvate Dehydrogenase Complex
pyruvate gets oxidized
Conversion of Pyruvate to acetyl-CoA
done by PDC (multi-enzyme complex)
occurs in the mitochondrial matrix
acetyl-coA is the fuel of the krebs cycle
irreversible at physiological conditions
involves a decarboxylation/oxidation of pyruvate to acetate in the form of a thioester, followed by the formation of acetyl CoA
Pyruvate + NAD+ + CoASH —> Acetyl-CoA + CO2 + NADH + H+
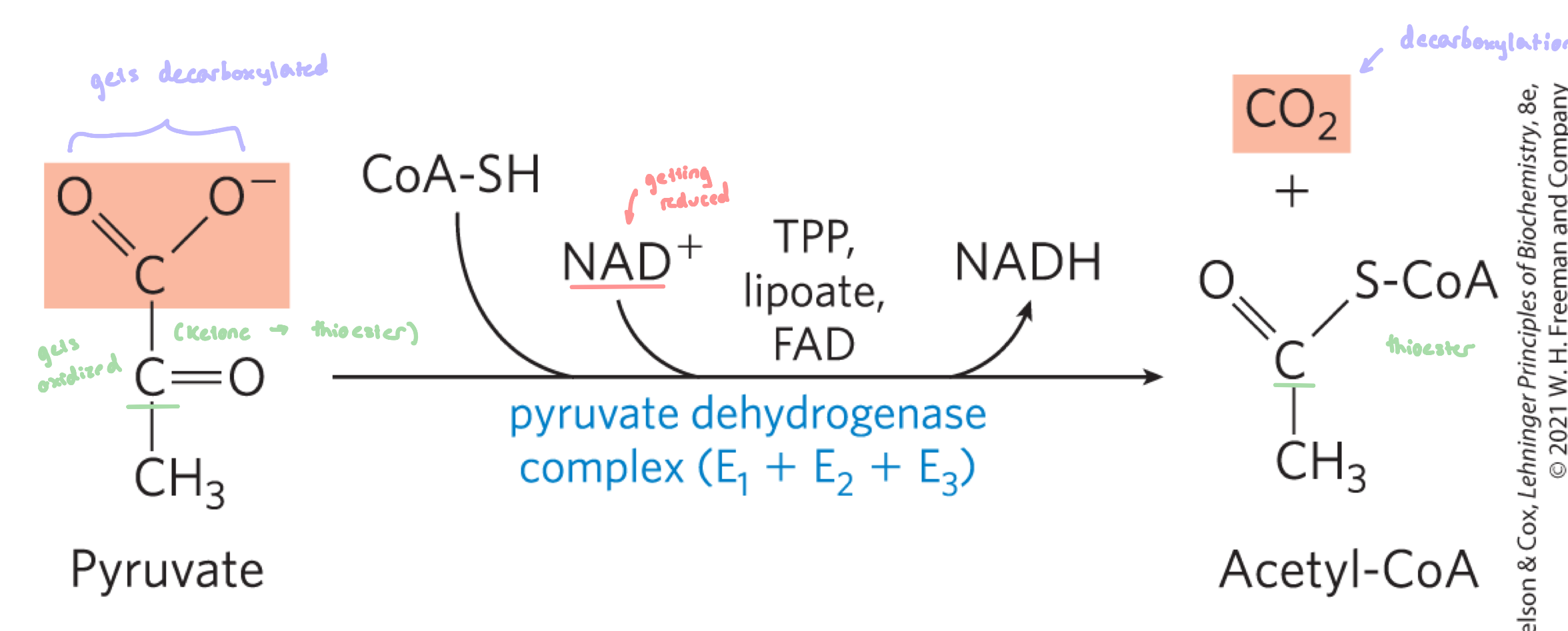
4º Structure of PDC
Complex is composed of many copies of the enzymes and cofactors
Enzymes:
E1: Pyruvate dehydrogenase
E2: Dihydrolipoyl transacetylase
E3: Dihydrolipoyl dehydrogenase
Cofactors:
thiamine pyrophosphate (TPP), bound to E1
lipoamide, bound to E2
NAD+, free
FAD, bound to E3
CoAsh, free
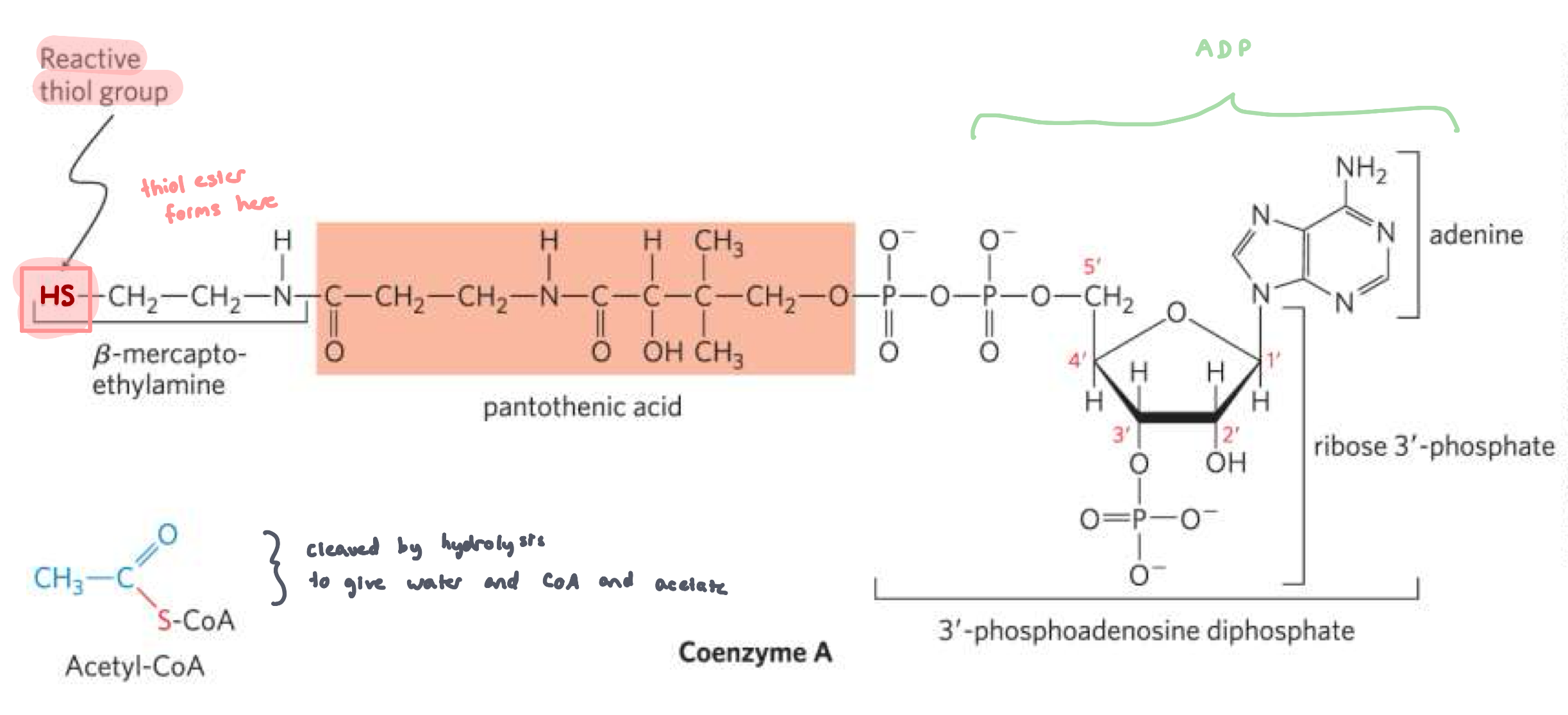
CoEnzyme A and Acetyl CoA
Coenzyme A: aka CoA or CoAsh
carrier of acyl groups
forms high energy thioester bonds
Acetyl CoA + H2O ⇌ Acetate + CoASH
∆Gº’ = -31 KJ/mol
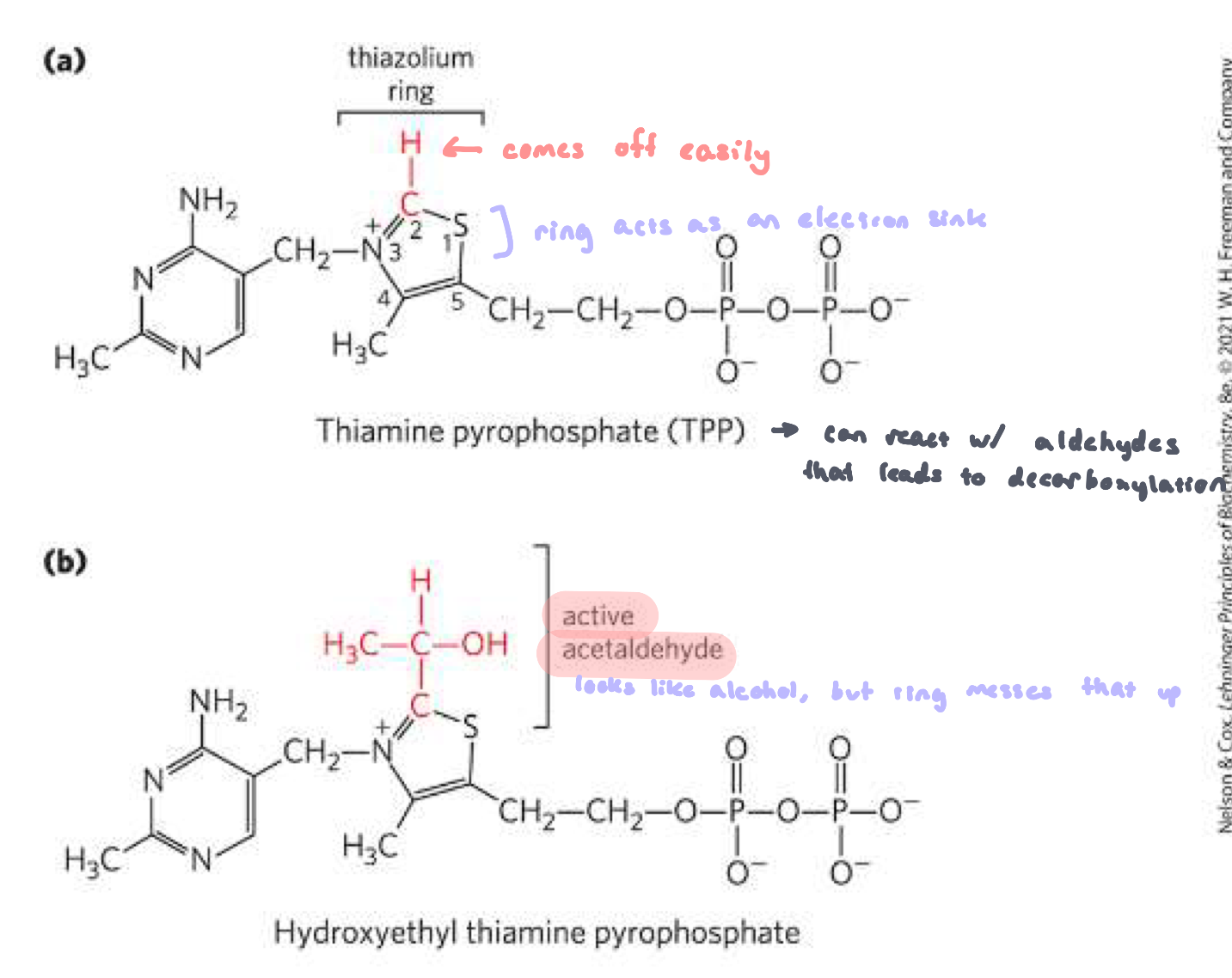
Thiamine Pyrophosphate (TPP) and hydroxyethyl TPP
derived from vitamin B1
forms a reactive carbanion
carries aldehyde
usually causes a decarboxylation
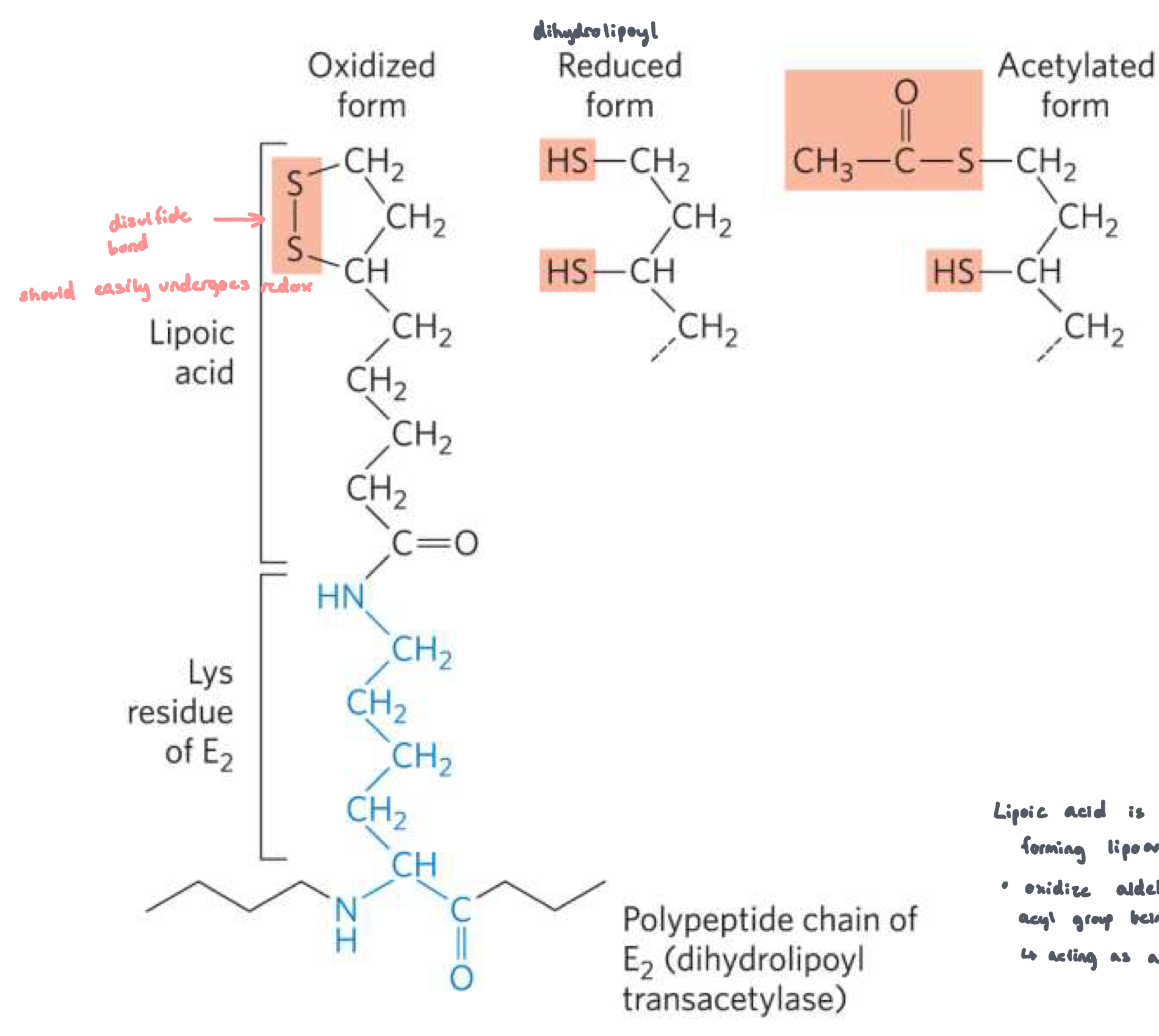
Lipoamide and Lipoic acid
lipoic acid is attached to a lysine R group, forming lipoamide
lipoamide is very flexible
oxidize aldehydes to acyl groups, resulting in the acyl group being bound via the disulfide group
acting as a robotic arm
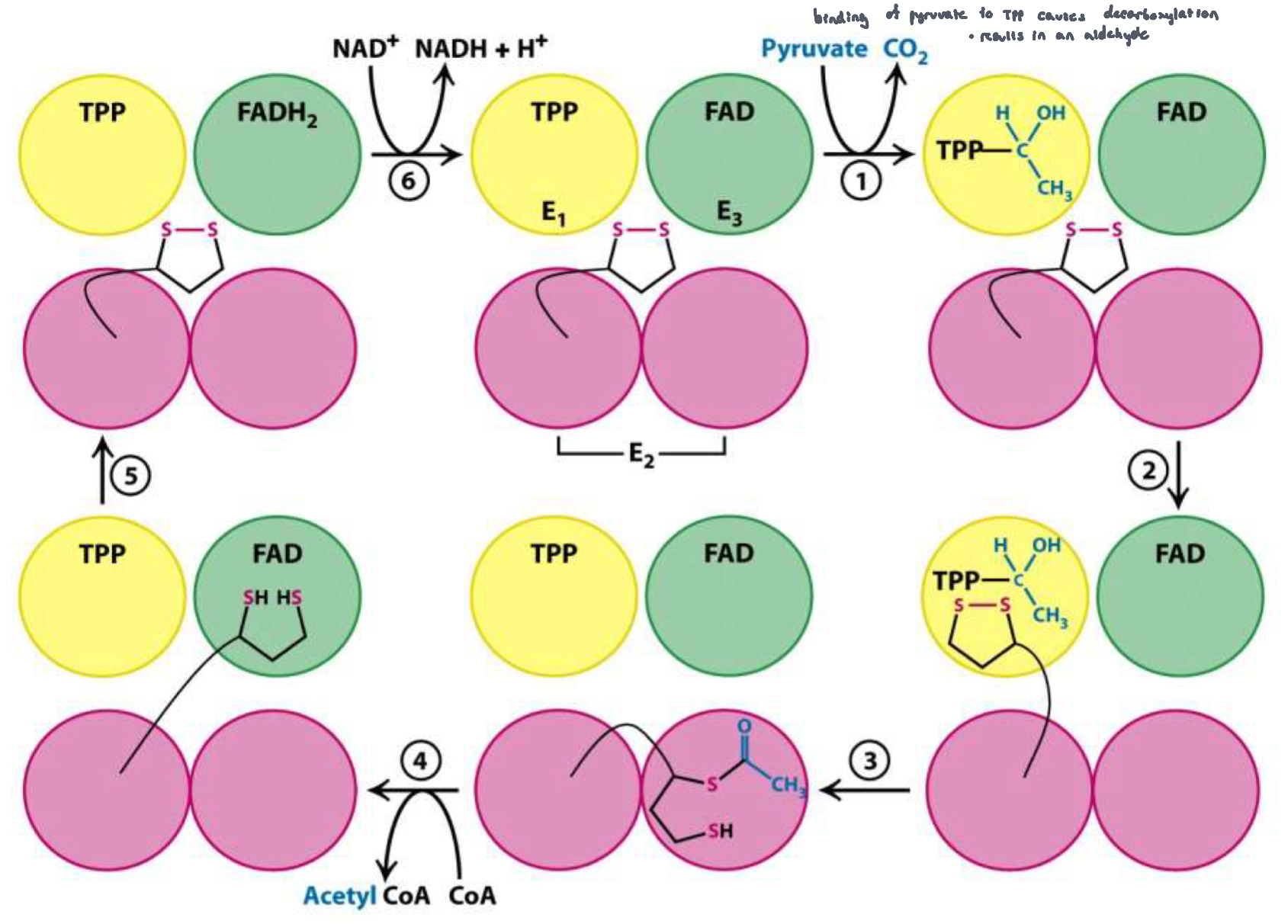
Mechanism of PDC
Pyruvate enters E1, binds to TPP and is decarboxylated to form the intermediate hydroxyethel, TPP
the oxidized limpoamide arm enters E1
the hydroxyethyl group is oxidized to an acetyl group, and is bound to the lipoamide arm. The arm is now reduced to a dihydrolipoyl group
the reduced arm carrying the acetyl group moves into E2 where the acetyl group is transferred to CoASH, forming acetyl CoA. Acetyl-CoA leaves the enzyme
the reduced lipoamide arms moves into E3, where it is reoxidized. In the process, FAD is reduced FADH2
NAD+ enters E3 and reoxidizes FADH2 back to FAD. NAD+ is reduced to NADH and H+ which leaves the enzyme
Back to step 1
Mechanism of PDC: Figure
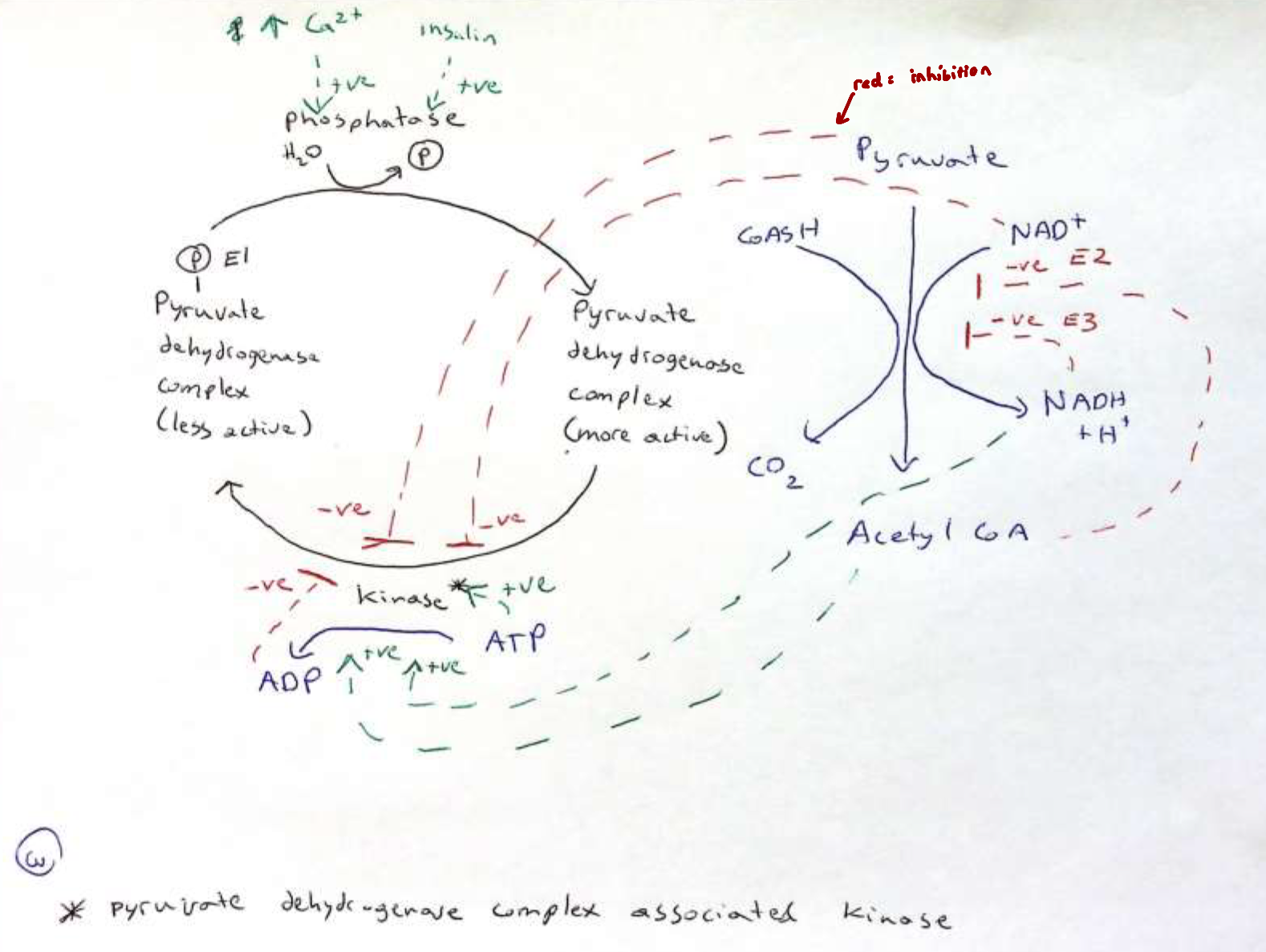
Regulation of the PDC
high conc. of acetyl CoA allosterically inhibits E2
high [NADH] allosterically inhibits E3
The main control is on E1, where phosphorylation by a kinase leads to inhibition of E1 and thus the complex
this kinase is the PDC associated kinase (PDCAK)
acetyl CoA, NADH and ATP all stimulate the kinase
pyruvate, NAD+ and ADP all inhibit the kinase
general phosphatases will gradually remove the phosphate from E1, allowing E1 to reset and become active
Cell signaling, such has high [Ca2+] and insulin activates the PDC associated phosphatase (PDCAP) that results in the rapid dephosphorylation of E1
Regulation of PDC: Figure
Krebs Cycle
metabolic hub of the cell
completely oxidizes acetyl CoA to CO2 , and in the process, generates high energy e- in the forms of NADH and FADH2 and GTP
source of many biological precursors
occurs in the mitochondrial matrix
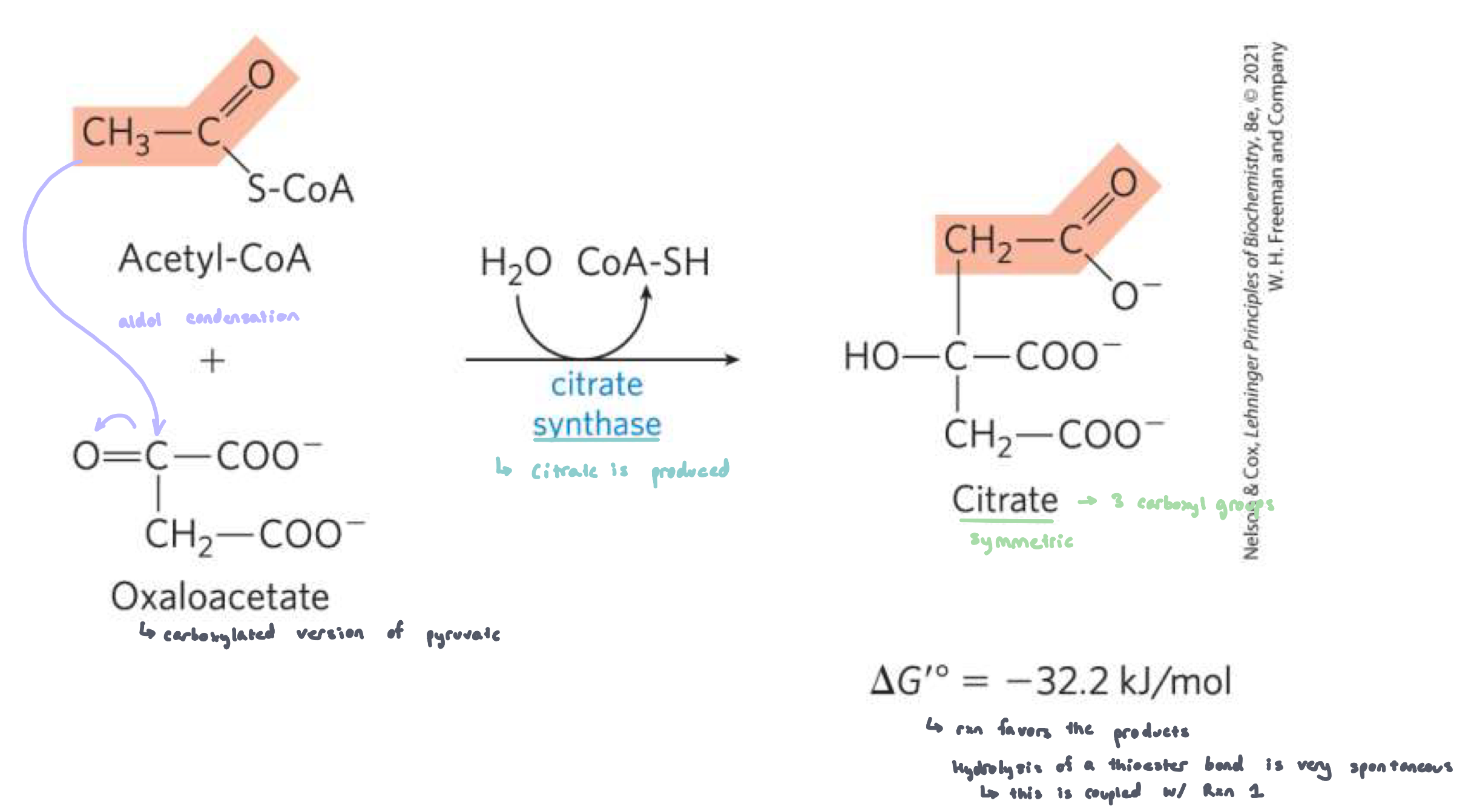
Reaction 1: Synthesis of Citrate from Acetyl CoA and Oxaloacetate (Citrate synthase)
citric synthase forms citrate by binding oxaloacetate to acetyl coA
going from C4 to C2
2 part process:
aldol condensation to form citryl CoA
hydrolysis of citryl CoA to citrate and CoASH (very favorable step)
Synthase vs Synthethase
Synthase: an enzyme catalyzing a synthetic reaction in which 2 units are joined without the direct participation of ATP (NTP)
Synthethase: same as above, but ATP (NTP) is directly required
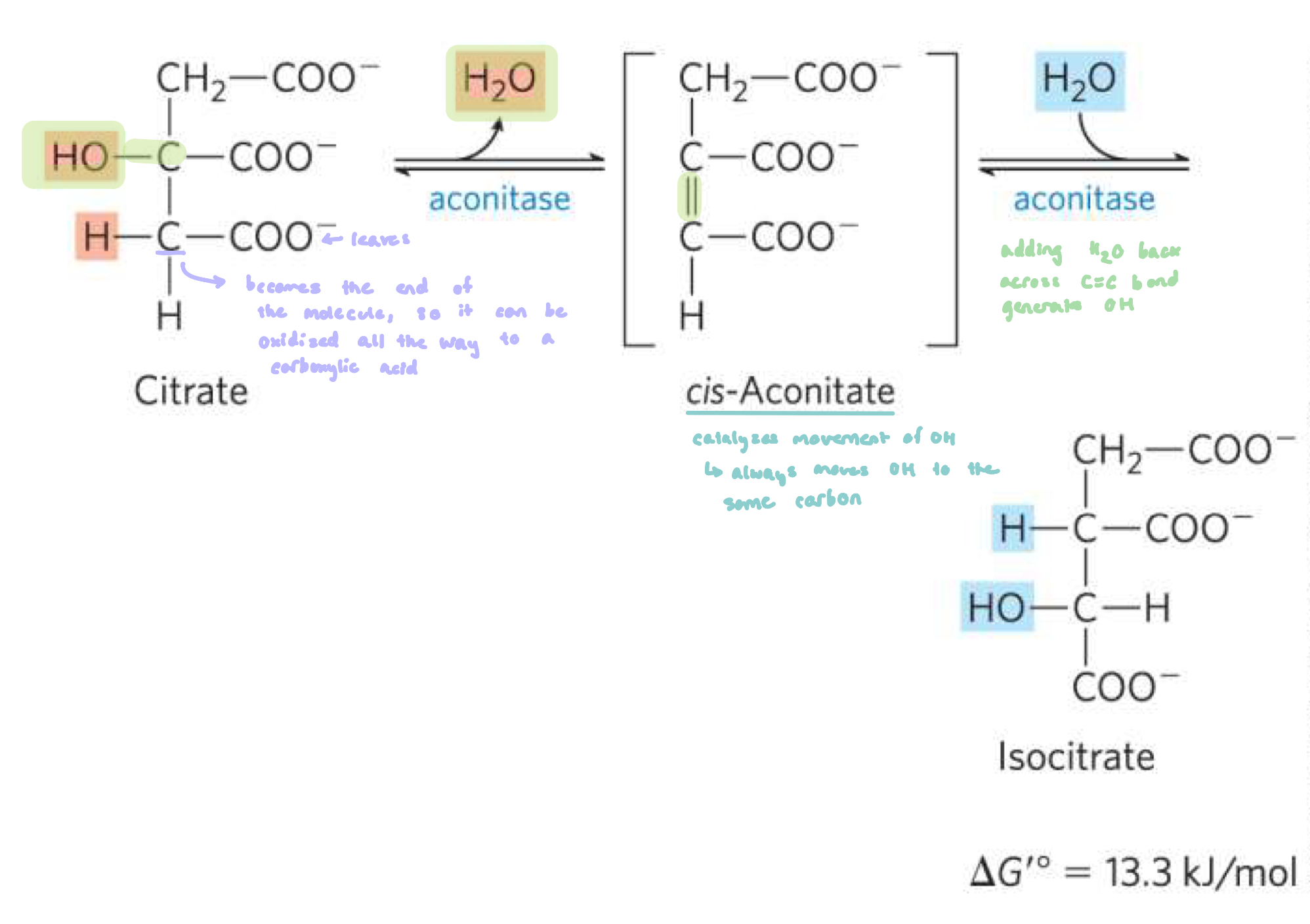
Reaction 2: Conversion of Citrate to Isocitate (Aconitase)
aconitase converts citrate to isocitrate
moving the OH group
dehydration reaction to form cis-aconitate
followed by a hydration step to generate iso-citrate
∆Gº’ > 0, but the reaction is driven by both reaction 1 and 3
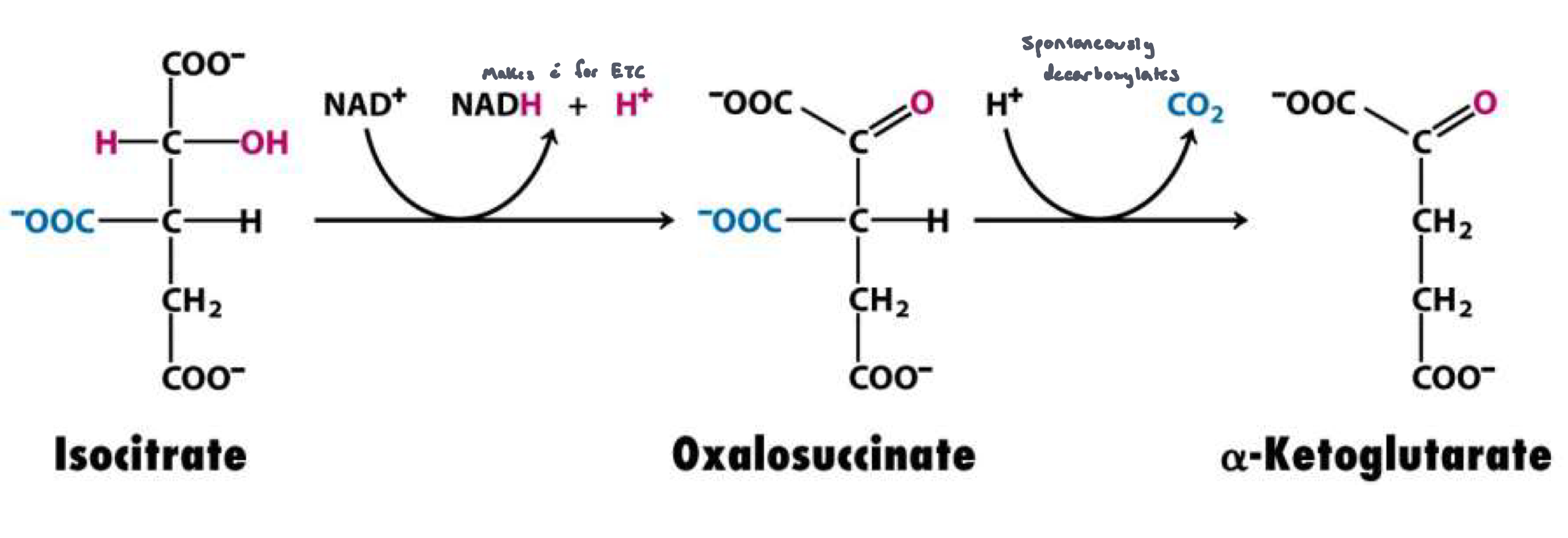
Reaction 3: Decarboxylation/Oxidation of Isocitrate to α-ketoglutarate (isocitrate dehydrogenase)
irreversible reaction
isocitrate is oxidized/decarboxylated to α-ketoglutarate, NADH and CO2 are produced
2 step process:
isocitrate is oxidized to ozalosuccinate, generating NADH
oxalosuccinate is decarboxylated to α-ketoglutarate spontaneously (5 carbons)
Note: the CO2 lost did not originate from the acetyl CoA that just entered the cycle
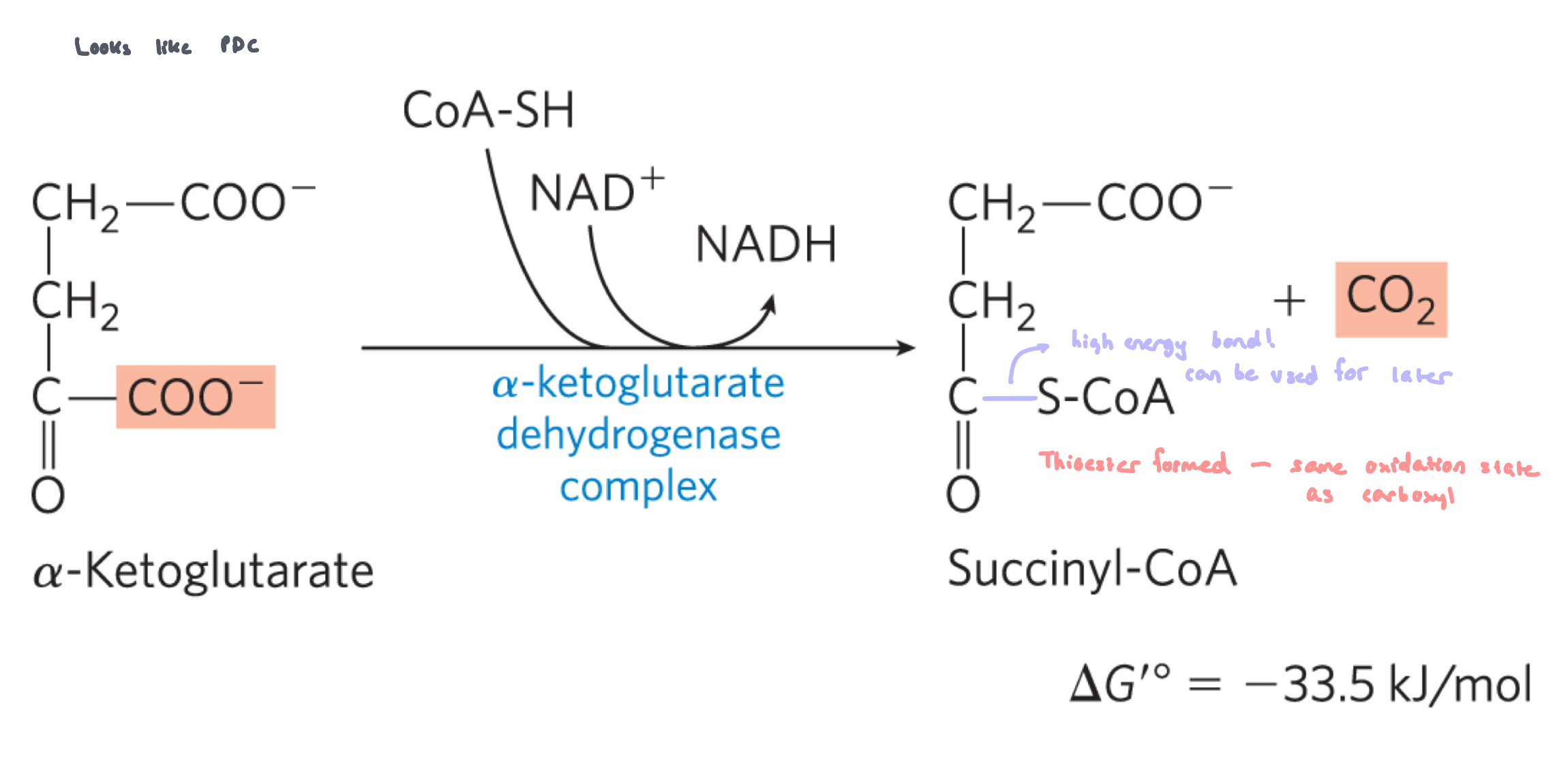
Reaction 4: Oxidation/Decarboxylation of ⍺-ketoglutarate to succinyl-CoA (⍺-ketoglutarate dehydrogenase complex)
irreversible reaction
⍺-ketoglutarate is decarboxylated/oxidized and bound to CoASH by ⍺-ketoglutarate dehydrogenase complex. This generates succinyl CoA, NADH and CO2
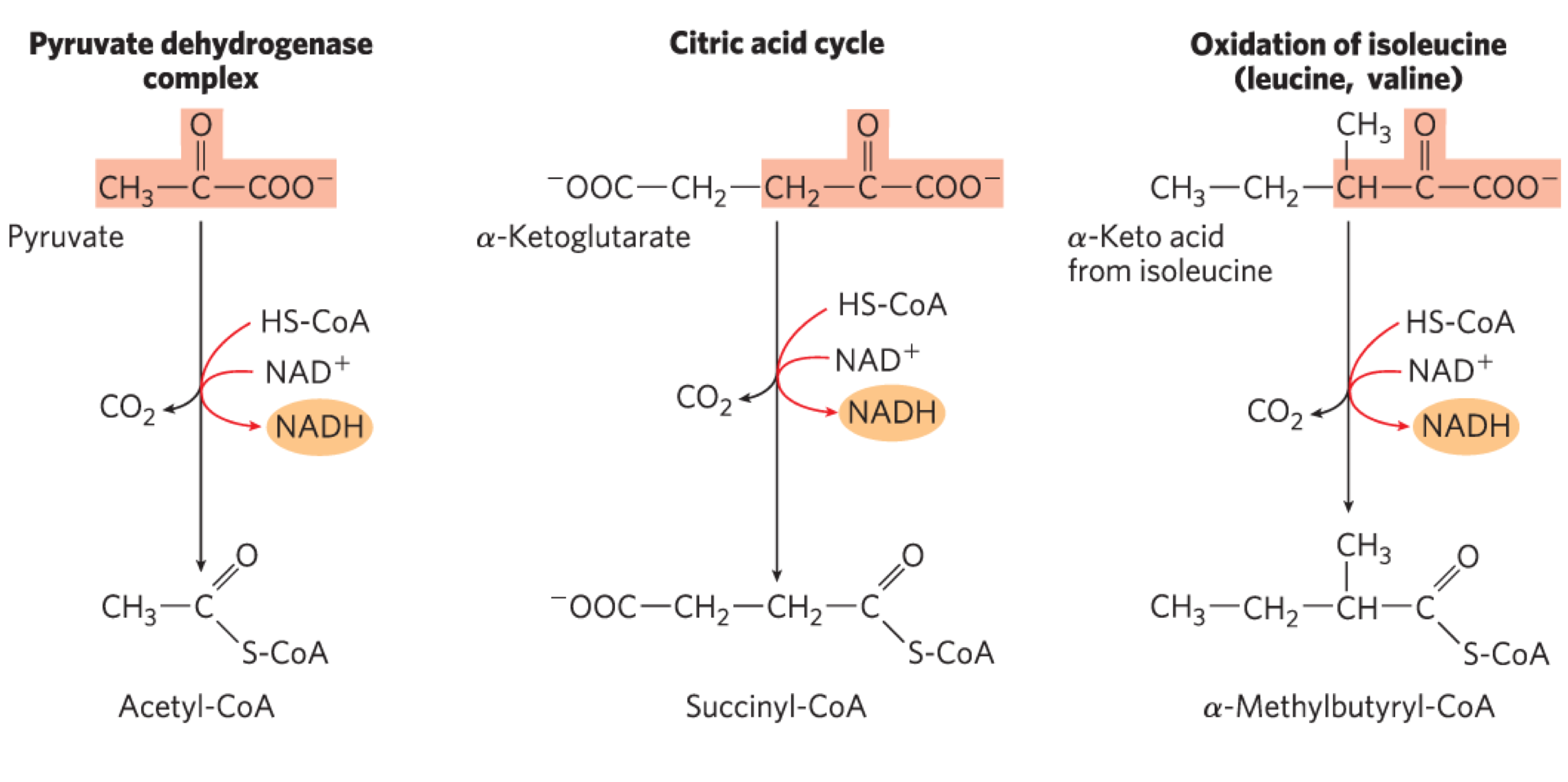
occurs by the same method as the PDC
same cofactors, similar E1 and E2 enzymes and identical E3 enzyme
back to 4 carbons
Conserved Mechanism for Oxidative Decarboxylation
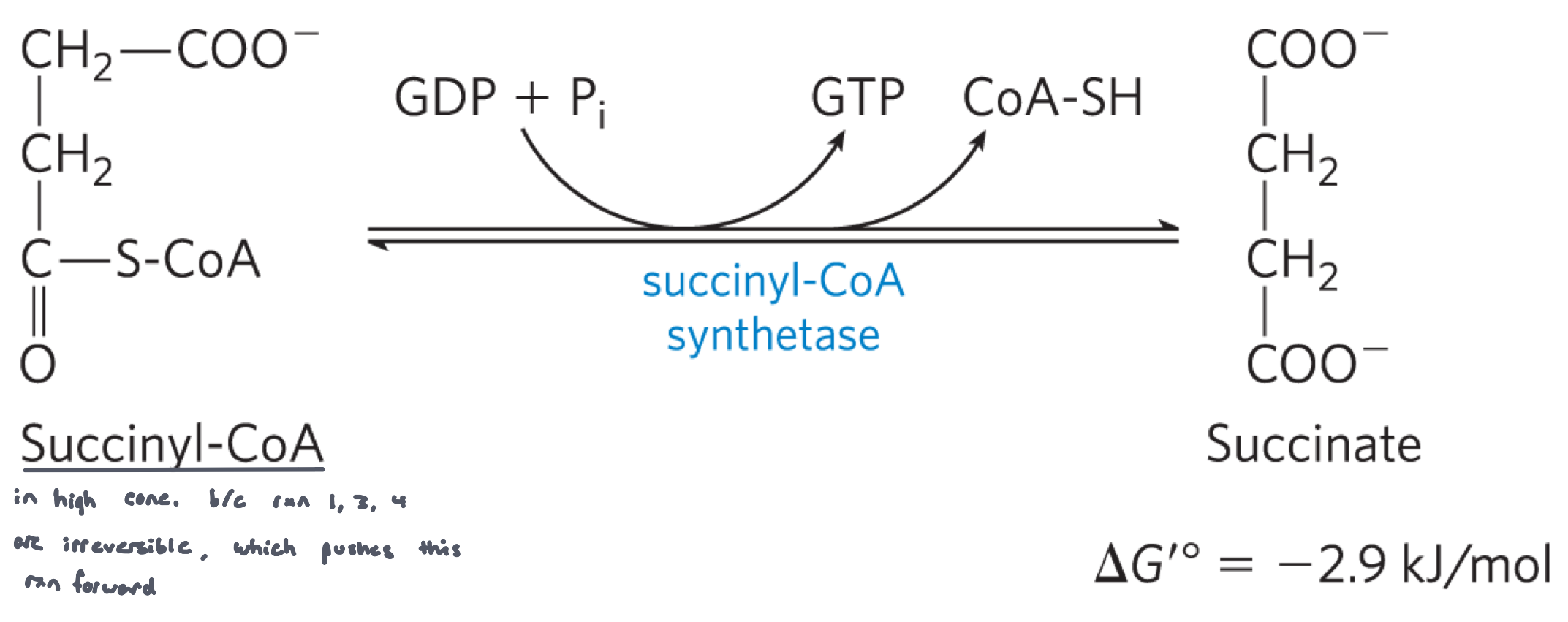
Reaction 5: Conversion of Succinyl CoA to Succinate with GTP generation (Succinyl CoA synthetase)
succinyl CoA synthetase converts succinyl CoA to succinate, generated GTP and CoASH
the reaction is driven by the negative ∆Gº’ in the cleavage of thioester bond
GTP can be converted to ATP by nucleoside diphosphate kinase
GTP +ATP⇌GDP + ATP
There are isoforms of succinyl CoA synthethase that use ADP instead of GDP
the next steps are involved in the regeneration of oxaloacetate
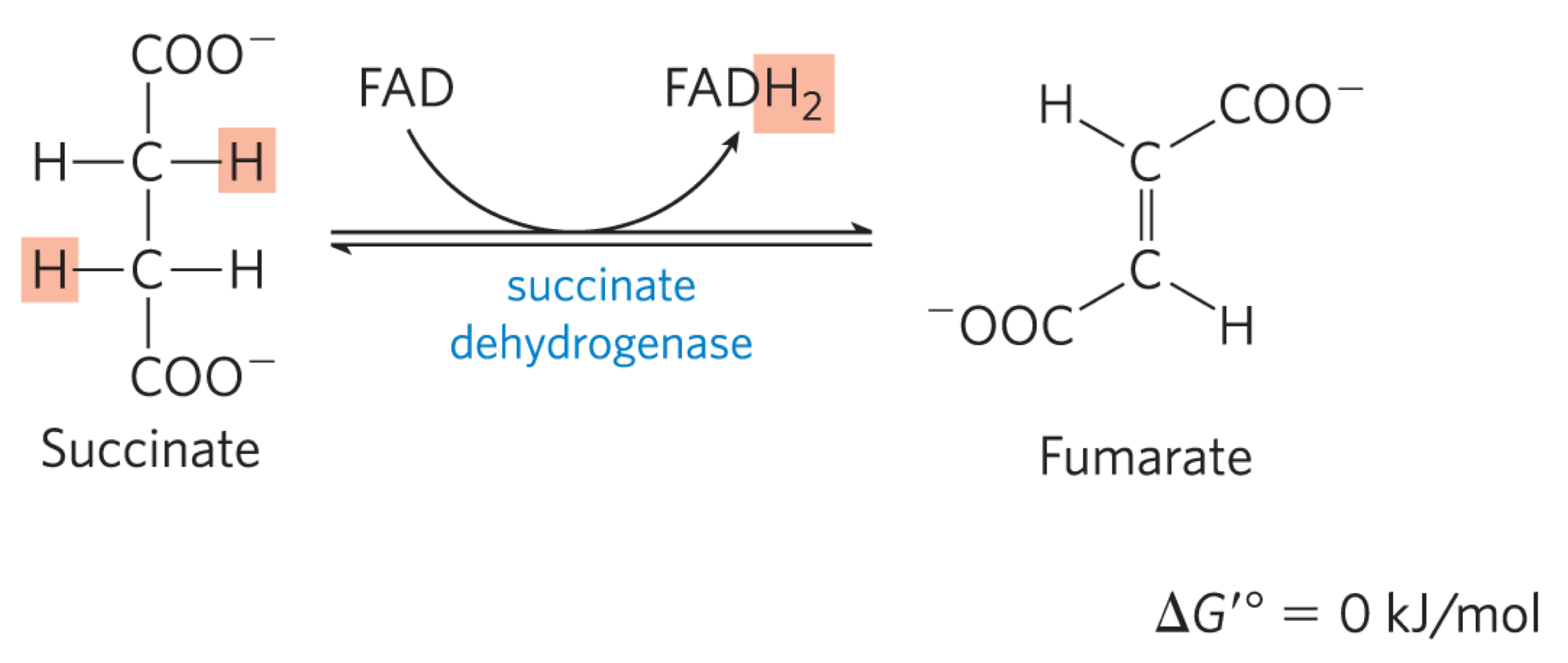
Reaction 6: Oxidation of Succinate to Fumarate (Succinate Dehydrogenase)
succinate dehydrogenase oxidizes succinate, generating FADH2 and fumarate (trans)
free energy change is not high enough to reduce NAD+
succinate dehydrogenase is part of complex 2
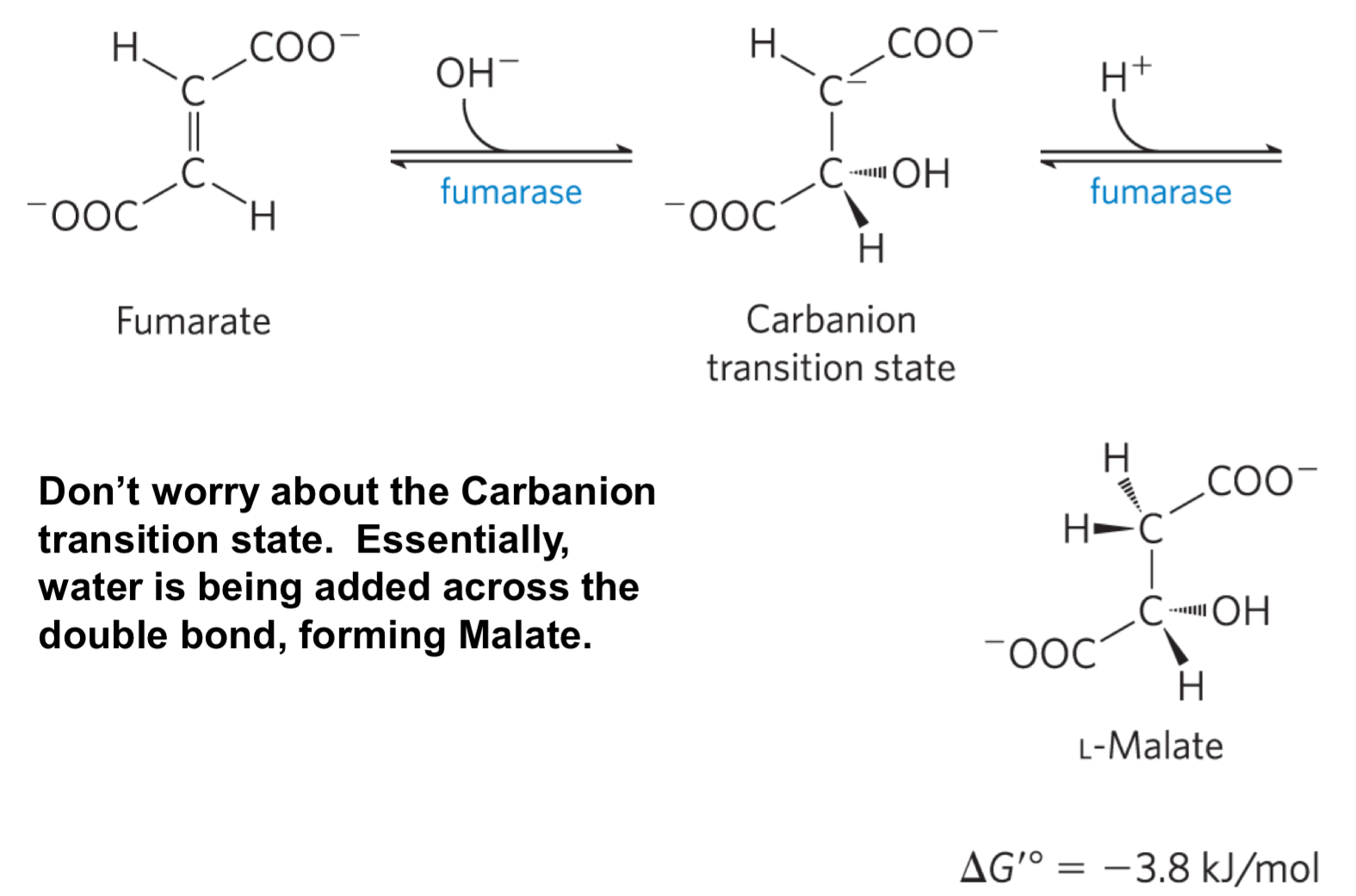
Reaction 7: Adding water across the double bond of fumarate to form malate (fumarase)
Fumarase adds water across the double bond, forming L-Malate
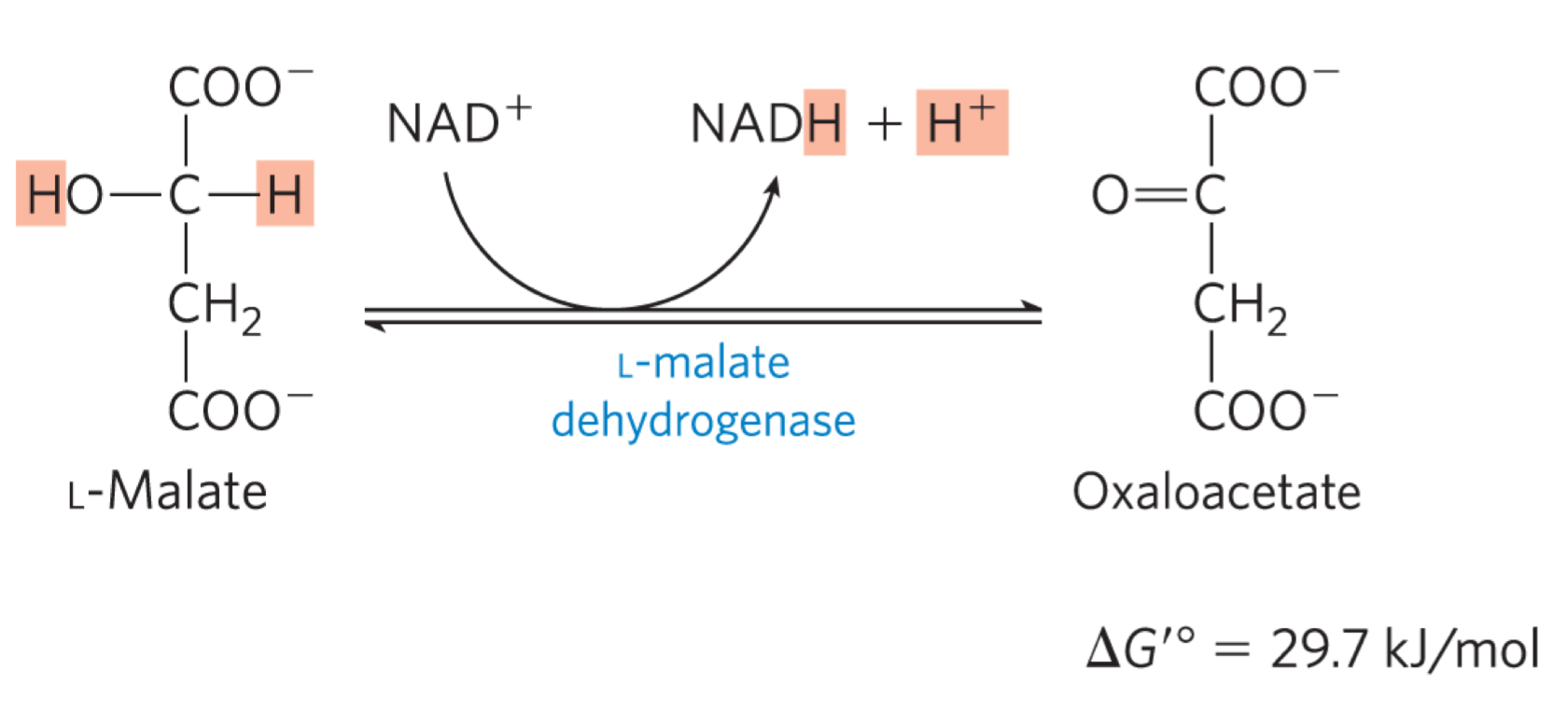
Reaction 8: Oxidation of Malate to Oxaloacetate (malate dehydrogenase)
malate dehydrogenase oxidizes malate to oxaloacetate, generating NADH
cyclic is complete
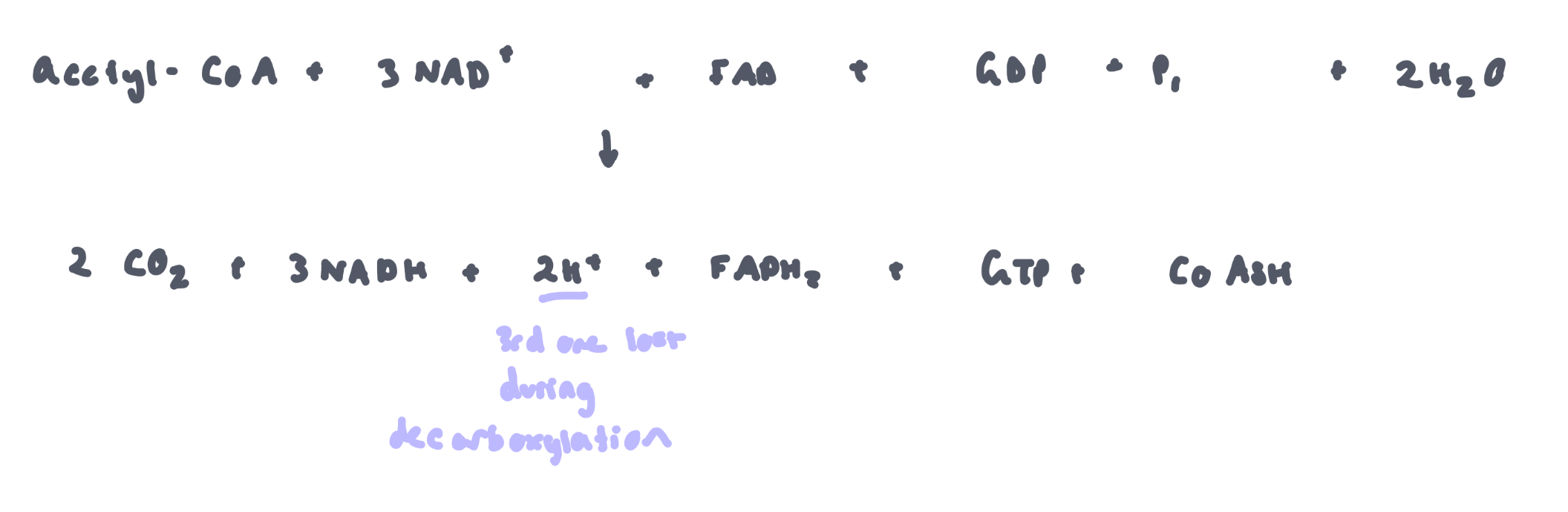
Overall (1 turn of Krebs Cycle)
Regulation of the Krebs Cycle
Allosteric regulation
Isocitrate dehydrogenase
stimulate by ADP
inhibited by NADH and ATP
⍺-ketoglutarate dehydrogenase complex
ATP, NADH and succinyl CoA all inhibit the enzyme
Optional (only in bacteria)
Citrate synthase
inhibited by ATP