elements, compounds, and mixtures; atomic structure and the periodic table
0.0(0)
Card Sorting
1/7
There's no tags or description
Looks like no tags are added yet.
Study Analytics
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
8 Terms
1
New cards
elements
made of only one type of atom
atoms that have the same number of protons
2
New cards
compound
two or more elements that have reacted with eachother and formed chemical bonds
3
New cards
mixtures
a substance made up of two or more elements or compounds that is not chemically bonded
4
New cards
question 1
NH4 has + charge
SO4 has 2- charge
you need two NH4 for every SO4
(NH4)2SO4
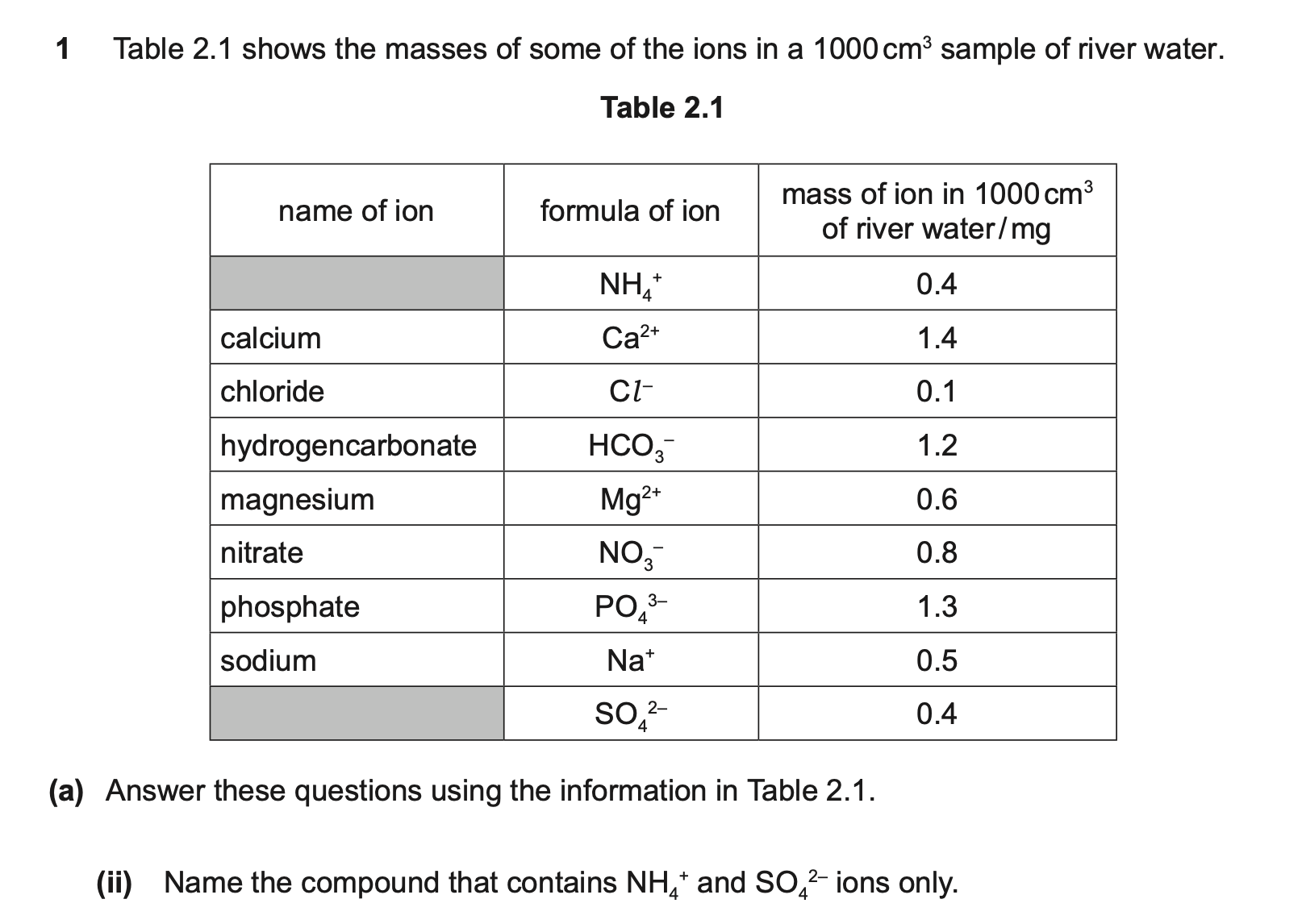
5
New cards
question 2
Al has 3+ so the electronic configuration is 2,8
N has 3- so the electronic configuration is 2,8

6
New cards
more metallic
if theres less valence electrons, it is more metallic because its easier to lose electrons and form ionic bonds
7
New cards
relative mass of an electron
1/1836
8
New cards
relative mass of a neutron and proton
1