Theme 2 Module 3+4
1/71
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
72 Terms
Flow of Genetic Information
Genome info encoded in genes-->DNA → transcribed into mRNA → translated into protein.
Why do proteins fold
Fold into 3D structures to give them diverse functions
Molecular components for translation
(ribosomal large and small subunits, mRNA, tRNA, energy and initiation elongation and termination factors)
tRNA role
Links mRNA codons to amino acids, able to transfer amino acids, from a pool of cytoplasmically situated amino acids to a growing polypeptide strand in a ribosome
Structure of tRNA
~70–90 nucleotides long (single RNA strand)
Hydrogen bonding between complementary nucleotide bases → 2D clover leaf also can be L shaped 3D
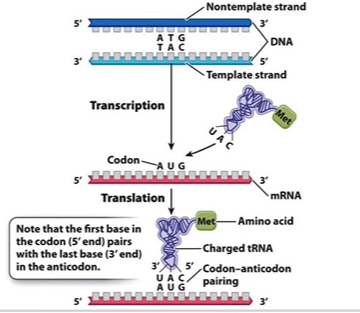
Anticodon loop for tRNA
3 bases complementary to mRNA codon (3’ → 5’)
CCA sequence
protruding attachment site for specific amino acid
CCA terminal A
actual point of attachment for amino acid during tRNA MOLECULE ACTIVATION
tRNA molecule activation
the process in which a specific amino acid is attached to its corresponding tRNA, facilitated by the enzyme aminoacyl-tRNA synthetase.
Aminoacyl tRNA synthestases
enzymes that catalyze the attachment of amino acids to their respective tRNAs, ensuring accurate protein synthesis.
How many aminoacyls
20 different ezymes one for each amino acid
Once aminoacyl bind to active site
Uses ATP hyhydrolysis to covalently attach correct amino acid → forms aminoacyl-tRNA (charged tRNA) and the enzyme releases the tRNA for protein synthesis.
How many tRNAs and why
Only ~45 tRNAs (not 64 like amino acids) because of codon wobble, allowing some tRNAs to pair with multiple codons
Wobble pairing
First two bases in codon pair very strictly with anticodon
Flexibility at 3rd codon base can wobble, meaning the same tRNA can sometimes read more then one codon that codes for the same amino acid
Prokaryotic translation
transcription & translation both in cytoplasm (can happen simultaneously)
Eukaryotic translation
two separate processes due to compartmentalization of DNA in nucleus and ribosomes in cytoplasm (transcription in nuclus and translation in ribosomes)
Intiation in prokayoties
No 5' cap Initiation at Shine-Dalgarno sequence (ribosome binding site, few bases upstream of AUG).
polycistronic mRNA
one mRNA molecule contains the instructions to make several different proteins (due to grouped genes in prokaryotic DNA)
Eukaryotic intaiation
Translation initiation complex forms towards the 5’ cap of the mRNA
Then scans along mRNA until AUG start codon.
Intiation complex machinery made of what
Initiation factors + two small ribosomal subunit + methionine-charged tRNA + mRNA for translation
Steps for intiation complexes
Initiation factors bind to 5' cap of messenger RNA (allows for small ribosomal subunit recruitment)
Same time, other imitation factor bind to tRNA that is charged with methionine
Partially assembled initiation complex will move along mRNA 5'-->3' until AUG encountered
Then the large subunit of ribosome is then able to bind to rest of initiation complex using energy from GTP hydrolysis
The next charged tRNA molecule can then join the ribosome
Ribosomal translation complex is assembles initiation factors released
Ribsome sites and functions
A site (Aminoacyl): entry site for charged tRNA.
P site (Peptidyl): holds growing polypeptide chain (also where methionine loc)
E site (Exit): where empty tRNA exits.
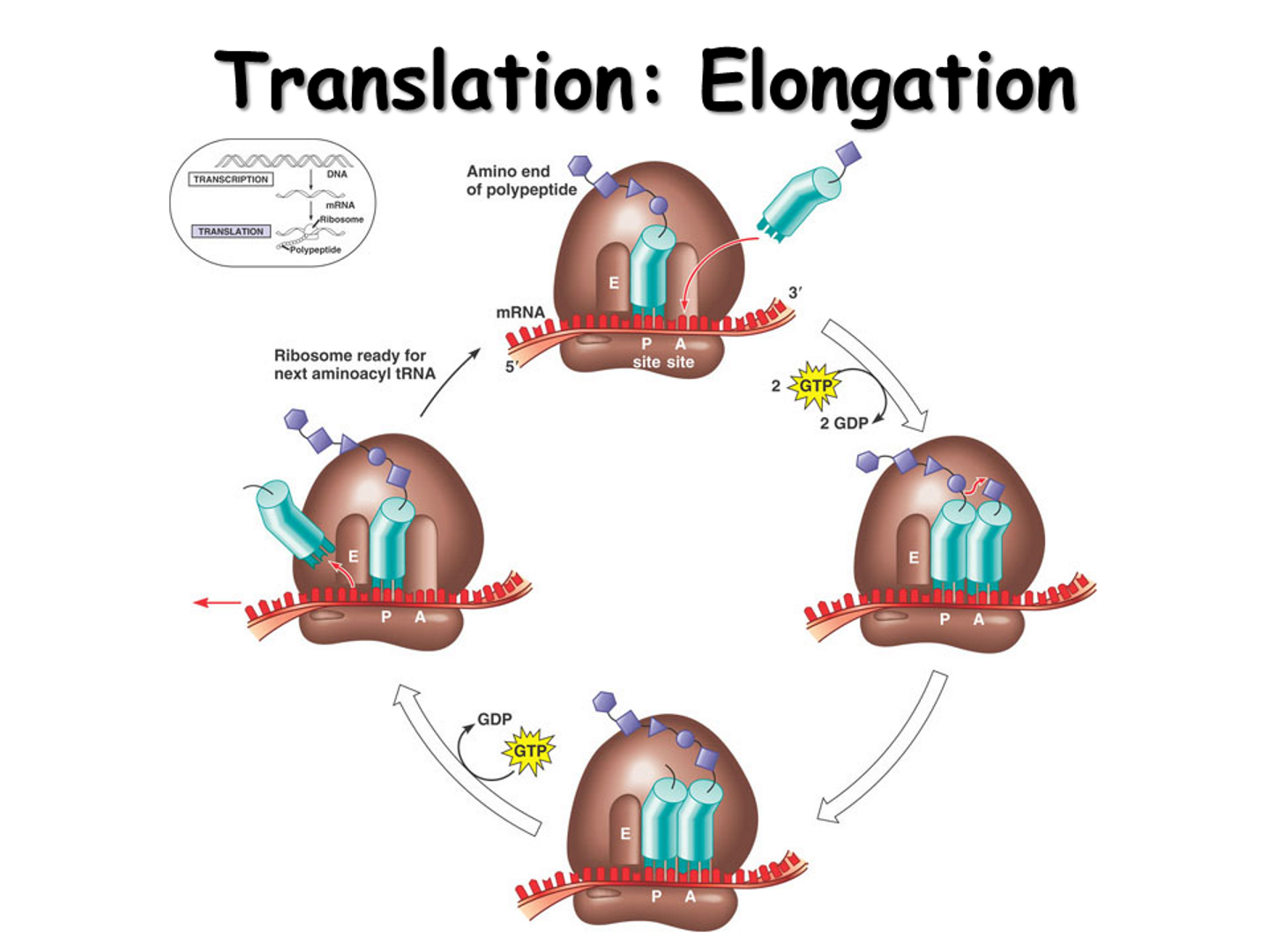
Elongation steps
First tRNA (methionine in in P-site)
Charged tRNA enters A site with elongation factor + GTP.
If codon-anticodon match → GTP hydrolyzed to release energy and lock in that tRNA
Peptidyl transferase (rRNA enzymatic function) forms peptide bond between amino acids in P site with new amino acid in A site
Polypeptide chain transfers to tRNA in A site.
Ribosome translocate (using GTP molecule)
Empty tRNA in P → E site (exits).
tRNA in A (with growing chain) → P site.
New codon now exposed in A site for next tRNA to enter
Termination
Ribosome reaches stop codon (UAA, UAG, UGA), no tRNA matches stop codon
Release factors (proteins bound to GTP) bind to A site.
Release factors functions
--> Trigger hydrolysis of bond between last amino acid and the tRNA
--> Use more GTP energy to make ribosome fall apart into subunits
All organisms use the same machinery
(ribosome large and small subunits, mRNA, tRNA, energy and initiation elongation and termination factors) only location differs
Beadule and Tatum
One Gene One ezyme hypothesis, using bread mold Neurospora crassa
What does the One-Gene-One-Enzyme hypothesis state?
Each gene encodes a single enzyme that controls a specific step in a metabolic pathway.
Why can Neurospora crassa grow on minimal medium?
It can synthesize all essential amino acids and vitamins using enzymes that convert simple nutrients into complex molecules.
What happens when a gene is mutated in Neurospora?
The fungus can’t make a specific enzyme → can’t produce a needed nutrient → can’t grow unless that nutrient is added externally.
In the arginine synthesis pathway, what are the steps and enzymes involved?
Precursor → (Enzyme 1) → Ornithine → (Enzyme 2) → Citrulline → (Enzyme 3) → Arginine
Why is arginine important in this experiment?
Mutant Neurospora strains that couldn’t make arginine would only grow if arginine (or a precursor) was supplied in the medium.
What was the purpose of growing mutants on different media (minimal, +ornithine, +citrulline, +arginine)?
To determine which step in the arginine synthesis pathway was blocked by each mutation.
What happened on arginine-supplemented medium?
Mutants grew normally → positive control (shows cells are alive if given the final product).
What happened on minimal medium?
No growth → mutation blocked arginine synthesis somewhere in the pathway.
What did growth with ornithine or citrulline indicate?
The mutant lacked the enzyme that worked before the step involving that compound.
What are the three mutant types identified by Srb & Horowitz?
Mutant | Missing Enzyme | Step Blocked |
|---|
arg1 | Enzyme 1 | Precursor → Ornithine |
arg2 | Enzyme 2 | Ornithine → Citrulline |
arg3 | Enzyme 3 | Citrulline → Arginine |
What conclusion did Srb & Horowitz reach?
Each gene controls the production of a specific enzyme → supports the One-Gene-One-Enzyme hypothesis.
Why was the hypothesis later modified?
Not all proteins are enzymes — genes can also code for structural or regulatory proteins.
What does the updated One-Gene-One-Polypeptide hypothesis state?
Each gene codes for one polypeptide chain, which may function alone or as part of a larger protein complex.
Example of a protein made from multiple polypeptides?
Hemoglobin — made of four polypeptide subunits, each coded by a different gene.
How many protein-coding genes are in the human genome?
About 20,000–25,000.
How can humans make over 100,000 different proteins from fewer genes?
Because of alternative splicing and post-translational modifications.
What is alternative splicing?
Process where one gene’s RNA can be cut and rearranged in different ways → creates multiple mRNAs → multiple proteins.
What are post-translational modifications?
Chemical changes to a protein after it’s made (e.g., phosphorylation, glycosylation) that alter its function.
What does this mean for the original hypothesis?
The basic idea is still true (genes → proteins), but one gene can produce multiple different proteins depending on how it’s processed.
What is the difference between the genome and the proteome?
Genome: ~20–25k protein-coding genes in humans.
Proteome: >1,000,000 proteins due to alternative splicing and PTMs.
How can one gene code for multiple proteins?
Through alternative splicing (different mRNAs from the same gene) and post-translational modifications (chemical changes after translation).
What is eukaryotic compartmentalization and why is it important?
Nucleus: Transcription, RNA processing.
Cytosol: mRNA translation by free or ER-bound ribosomes.
ER & Golgi: Protein folding, modifications, secretion.
Importance: Allows precise regulation of cellular processes; the proteome changes in response to development or environmental signals.
Describe the flow of genetic information from DNA to functional protein.
DNA → transcription → pre-mRNA
RNA processing → mature mRNA exported to cytosol
Translation → polypeptide synthesized in cytosol or ER
Post-translational modifications → functional protein
How do alternative splicing and PTMs contribute to proteomic complexity?
They allow a single gene to produce multiple functional proteins with different activities, locations, or interactions.
How do cells detect changes in their environment?
Stimuli are detected by sensor proteins, which trigger cellular responses through signaling pathways.
Give an example of a stimulus-response pathway involving glucose.
Stimulus: Increase in blood glucose
Sensor: Pancreatic beta cells detect glucose
Effector: Beta cells secrete insulin protein
Target: Cells with insulin receptors absorb glucose → lowers blood glucose
Where does most glucose absorption occur?
In the microvilli of the small intestine; absorbed glucose enters the bloodstream for distribution.
How do pancreatic beta cells respond to increased glucose?
They adjust insulin synthesis and secretion to regulate blood glucose levels.
How is insulin biosynthesis regulated?
At both transcriptional and translational levels; glucose metabolism stimulates insulin gene expression.
Where is insulin synthesized in beta cells?
Dense rough ER (RER) → site of translation and folding.
Describe the difference between preproinsulin and mature insulin.
Preproinsulin: 110 amino acids; contains a signal sequence directing it to the RER.
Mature insulin: 51 amino acids (A chain = 21 aa, B chain = 30 aa); formed after cleavage and folding (PTMs)
Steps in insulin maturation:**
Preproinsulin synthesized, signal sequence directs to RER
Signal sequence cleaved → proinsulin
Proinsulin folds and forms 3 disulfide bonds (chaperone-assisted)
Proinsulin cleaved in Golgi → mature insulin (A + B chains) + C-peptide released
Mature insulin is functional and can bind insulin receptors
Why are post-translational modifications (PTMs) important?
PTMs increase protein diversity and regulate activity, interactions, stability, and localization
Name common types of PTMs.
Cleavage (e.g., insulin maturation)
Folding & disulfide bonds
Covalent modifications:
Phosphorylation (Ser, Thr, Tyr)
Methylation
Acetylation
What type of receptor binds insulin?
Receptor tyrosine kinase (RTK)
Describe the mechanism of insulin receptor activation.
Insulin binds → receptor dimerizes
Autophosphorylation activates cytoplasmic kinase domains
Activated receptor phosphorylates downstream proteins
Glucose transporter proteins activated → glucose enters cells
What is signal amplification?
One insulin molecule triggers a large intracellular response, increasing efficiency of glucose uptake.
What are feedback loops in signaling?
Positive feedback: maintain signal
Negative feedback: terminate signal
Double-negative feedback: inhibitor of inhibitor → fine regulation
What is alternative splicing?
One pre-mRNA can be spliced in multiple ways → multiple mRNAs → multiple protein isoforms.
What are exons and introns?
Exons: Retained in mature mRNA → code for protein
Introns: Removed during splicing
What enzyme complex mediates splicing?
Spliceosome
Why is alternative splicing important?
Produces tissue-specific proteins
Increases proteome complexity
Helps regulate gene expression
Example – insulin receptor isoforms:**
Skeletal muscle: Exon 11 removed → high-affinity receptor → efficient glucose uptake
Liver: Exon 11 retained → low-affinity receptor → slower glucose uptake
How can changes in splicing or PTMs lead to disease?
Misprocessed proteins → nonfunctional → disease
Example: Misprocessed insulin → cannot bind receptor → hyperglycemia → diabetes
Incorrect insulin receptor isoform → poor glucose uptake → systemic issues