Chemistry - Unit 2: Atoms, Elements & Compounds
1/41
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
42 Terms
what are elements?
substances that cannot be broken down into simpler substances by chemical methods
what are compounds?
a substance containing 2 or more elements chemically combined together
what are mixtures?
a mixture consists of 2 or more substances that are not chemically combined together
describe the structure of an atom
a central nucleus containing neutrons and protons surrounded by electrons in shells
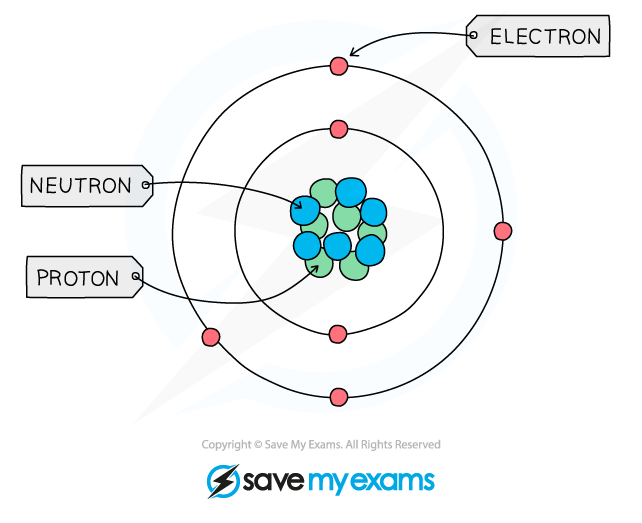
state the relative charge of a proton.
-1
state the relative charge of a neutron.
0
state the relative charge of an electron.
-1
state the relative mass of a proton.
1
state the relative mass of a neutron.
1
state the relative mass of an electron.
1/1840
define proton/atomic number
number of protons in the nucleus of an atom
define nucleon/mass number
total number of protons and neutrons in the nucleus of an atom
how to determine the electronic configuration of an element?
electrons orbit the nucleus in shells and each shell has a different amount of energy associated with it
the further away from the nucleus, the more energy a shell has
electrons fill the shell closest to the nucleus and when a shell becomes full of electrons, additional electrons have to be added to the next shell
the first shell can hold 2 electrons while the second and third shell can hold 8 electrons
the outermost shell of an atom is called the valence shell and an atom is much more stable if it can manage to completely fill this shell with electrons
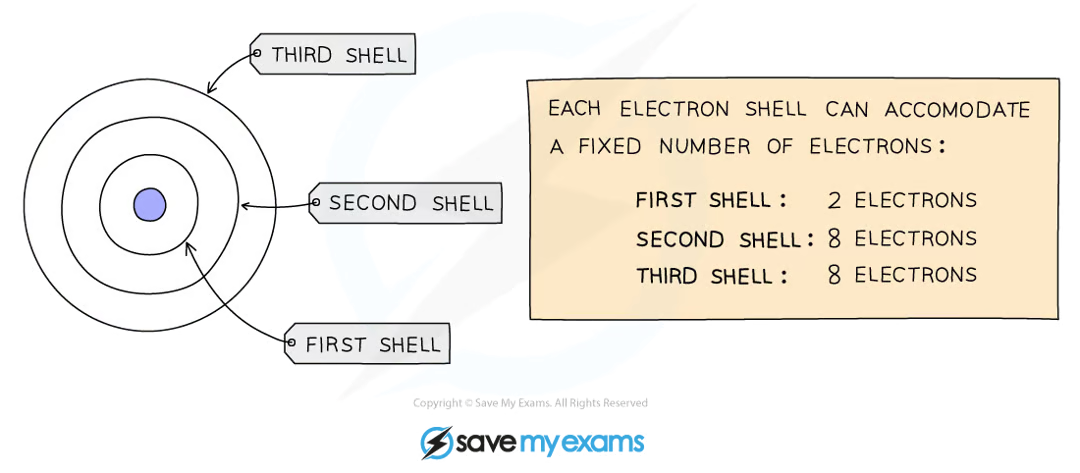
what do group VIII noble gases have?
a full outer electron shell
what is the number of outer shell electrons equal to?
the group number in Groups I to VII
what is the number of occupied electron shells equal to?
the period number
what are isotopes?
different atoms of the same element that have the same number of protons but different numbers of neutrons
why do isotopes of the same element have the same chemical properties?
they have the same number of electrons and therefore the same electronic configuration
how to calculate the relative atomic mass of an element from the relative masses and abundances of its isotopes
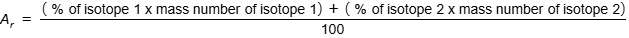
how to interpret symbols for atoms and ions
in a periodic table, the larger number next to the element is the mass number and the smaller number next to the element is the atomic number (most cases mass number is on top and atomic number is on the bottom)
atomic number determines the number of protons and electrons
mass number determines Ar of element and total number of neutrons and protons in the nucleus
number of neutrons can be determined by subtracting the atomic number from the mass number
for ions, a charge will be written next to the element. This charge is used to determine whether the ion is a cation or anion.
If the charge is (+), the ion is positive/cation and an electron has been lost.
If the charge is (-), the ion is negative/anion and an electron has been gained.
how are positive ions/cations formed?
positive ions/cations are formed when a metal atom loses electrons to gain a full outer shell of electrons in order to become more stable
how are negative ions/anions formed?
negative ions/anions are formed when a non-metal atom gains electrons to achieve a full outer shell of electrons in order to become more stable
describe a giant laittice structure of ionic compounds
a regular arrangement of alternating positive and negative ions
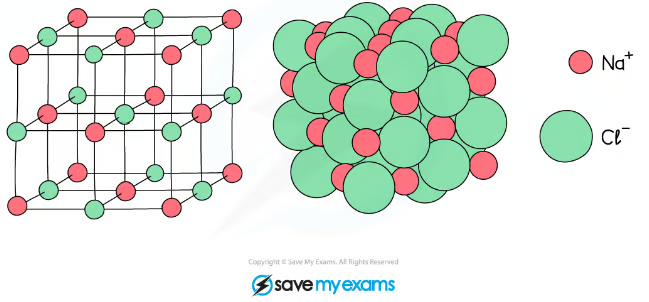
what is an ionic bond?
strong electrostatic forces of attraction between oppositely charged ions
describe the formation of ionic bonds between ions of metallic and non-metallic elements
metals have to lose electrons to become cations while nonmetals have to gain electrons to become anions
in the formation of ionic bonds, metals donate electrons to nonmetals. after the transfer, the anions and cations are held together by a strong electrostatic attraction, forming a stable compound.
The best way to visualise this process is through the dot-and-cross diagram:
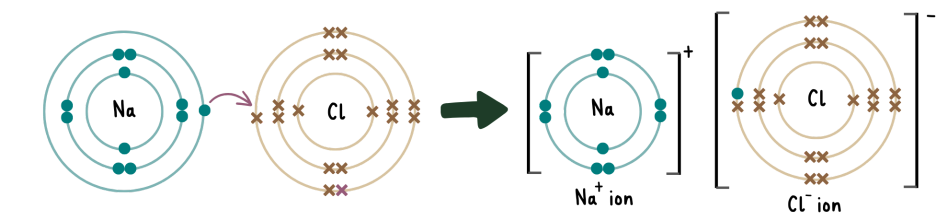
what are the properties of ionic compounds?
high melting and boiling points
good electrical conductivity
explain in terms of structure and bonding why ionic compounds have high melting and boiling points
ionic compounds have a giant lattice structure with strong electrostatic forces of attraction between oppositely charged ions so a lot of energy is needed to overcome them.
explain in terms of structure and bonding why ionic compounds have the inability to conduct electricity in solid form
ions are in fixed positions within the lattice and are unable to move as freely moving charged particles like electrons or ions need to be present for electrical current to flow
explain in terms of structure and bonding why ionic compounds have the ability to conduct electricity in molten or aqueous form
in a molten or aqueous state, ionic compounds have free moving ions that can carry a charge
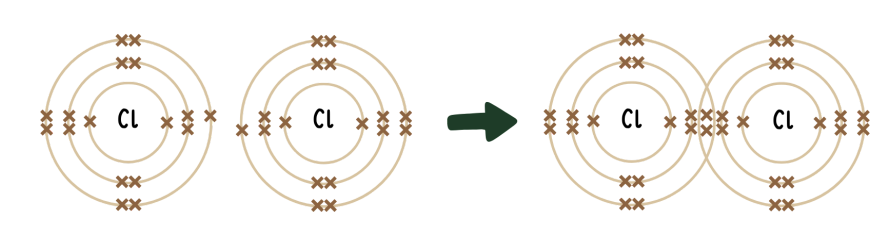
how are covalent bonds formed?
covalent bonds are formed when a pair of electrons is shared between 2 nonmetal atoms leading to noble gas electron configuration
this process can be shown visually using the dot-and-cross diagram method
what are the properties of simple molecular compounds?
low melting and boiling points
inability to conduct electricity
explain in terms of structure and bonding why simple molecular compounds have low melting and boiling points
simple molecular compounds have a simple molecular structure with weak intermolecular forces between molecules so little energy is required to overcome them.
explain in terms of structure and bonding why simple molecular compounds have the inability to conduct electricity
there is an absence of ions (charged particles) to carry any charge in simple molecular compounds
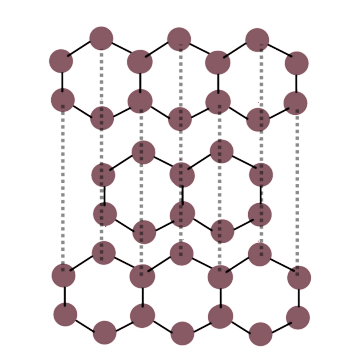
describe the giant covalent structure of graphite
in graphite, each carbon atom is covalently bonded to 3 other carbons.
its structure consists of layers of hexagonal rings with no covalent bonds between the layers.
the layers are connected by weak intermolecular forces, meaning the layers can slide over each other resulting in graphite being soft.
one electron from each carbon atom is delocalised, meaning graphite can conduct electricity as the delocalised electrons carry charge.
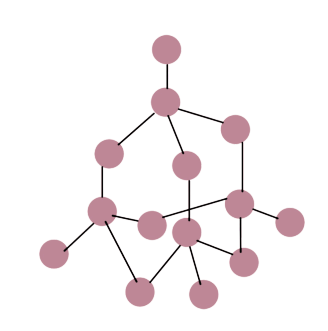
describe the giant covalent structure of diamonds
in diamond, each carbon atom is covalently bonded to 4 other carbon atoms
its structure is arranged in a tetrahedral 3D shape.
diamonds are very hard due to many strong covalent bonds
can’t conduct electricity as there are no delocalised electrons
describe the structure of silicon (IV) dioxide [SiO2]
silicon dioxide (a.k.a silica), is the main component of sand
each silicon atom is covalently bonded to 4 oxygen atoms
each oxygen atom is covalently bonded to 2 silicon atoms
thus, the formula is SiO² (as Si²O⁴ simplified is SiO²)
a tetrahedron is formed with one silicon atom and four oxygen atoms, similar to diamond
what are the uses of graphite?
lubricant
electrode
what are the uses of diamond?
cutting tools
why is graphite used as a lubricant
graphite has weak intermolecular forces of attraction between each layer of carbon atoms which slide past each other when force is applied
why is graphite used as an electrode
graphite has free moving electrons
why is diamond used in cutting tools?
due to its hardness through its tetrahedral, rigid arrangement of atoms within its giant covalent structure with many strong covalent bond between carbon atoms.
similarities between diamonds and SiO2
in SiO2 each silicon atom is covalently bonded to 4 oxygen atoms and in diamond, each carbon atom is covalently bonded to 4 carbon atoms
high melting and boiling points — both have strong covalent bonds
atoms bonded in a tetrahedral arrangement — both structures are very hard and rigid