Crystallisation
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
What is Crystallization and What is its Purpose?
Crystallization is the process of generating crystalline solids from a liquid (solution or melt).
The primary purposes of crystallization include:
Retrieving dissolved solids in crystalline form from a solution.
Purifying crystalline solids through recrystallization.
Producing solids with controlled particle size and crystal shape.
Principles for Exceeding the Saturation-Dissolution Curve
To initiate crystallization, the solution must exceed its saturation concentration. This can be achieved through:
Evaporation Crystallization:
Evaporating part of the solvent increases the solute concentration beyond saturation.
Cooling Crystallization:
Decreasing the temperature reduces solubility, moving the solution into the oversaturated region.
Vacuum Crystallization:
Reducing pressure causes simultaneous evaporation and cooling, leading to oversaturation.
Melt Crystallization:
Involves solidifying a melt into crystalline form.
Replacement Crystallization:
Adding a precipitating agent to induce crystallization.
Phases of Crystallization
Nucleation:
The initial formation of crystal seeds.
Happens either spontaneously or through external seed addition.
Crystal Growth:
Crystals grow from the seeds under controlled conditions of oversaturation.
Homogeneous and Heterogeneous Nucleation
Homogeneous Nucleation:
Crystals form spontaneously in a supersaturated solution without external impurities.
Heterogeneous Nucleation:
Crystals form around foreign particles (e.g., container walls, impurities).
Setup and Working Principle of Crystallization Evaporators
With Inner Heating:
Solution is pumped through an evaporator with heated pipes.
Vapor is removed, and crystals form in a reflux pipe. Because the solution becomes more concentrated and reaches the saturation conc., crystals are formed.
Crystals grow and sediment at the bottom for extraction. Then the crystal slurry leaves the crystallizer through a valve.
Part of the vapour is recondenced and sent back as a reflux.
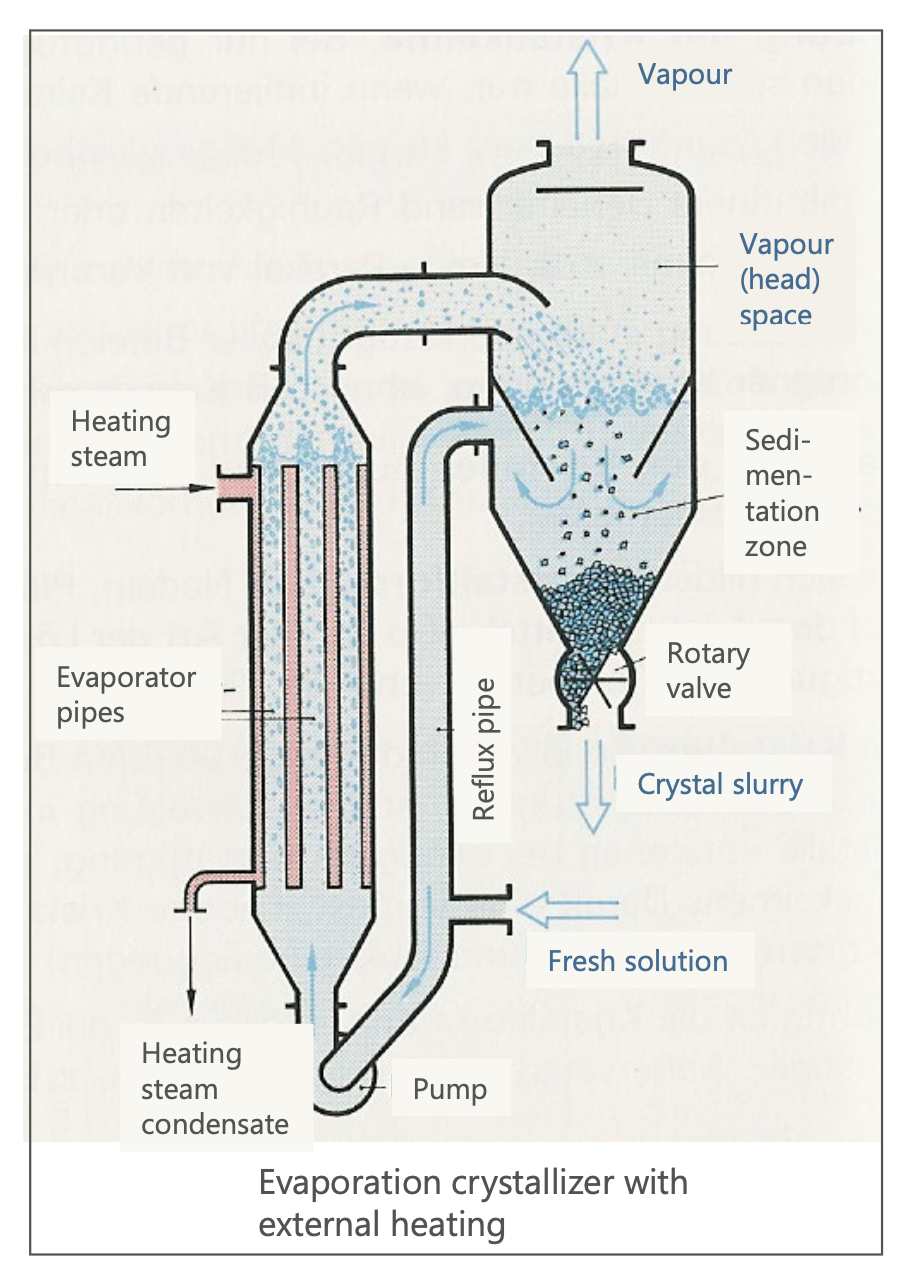
With Outer Heating:
Similar principle but uses external heat exchangers.
Suitable for viscous solutions with forced recirculation.
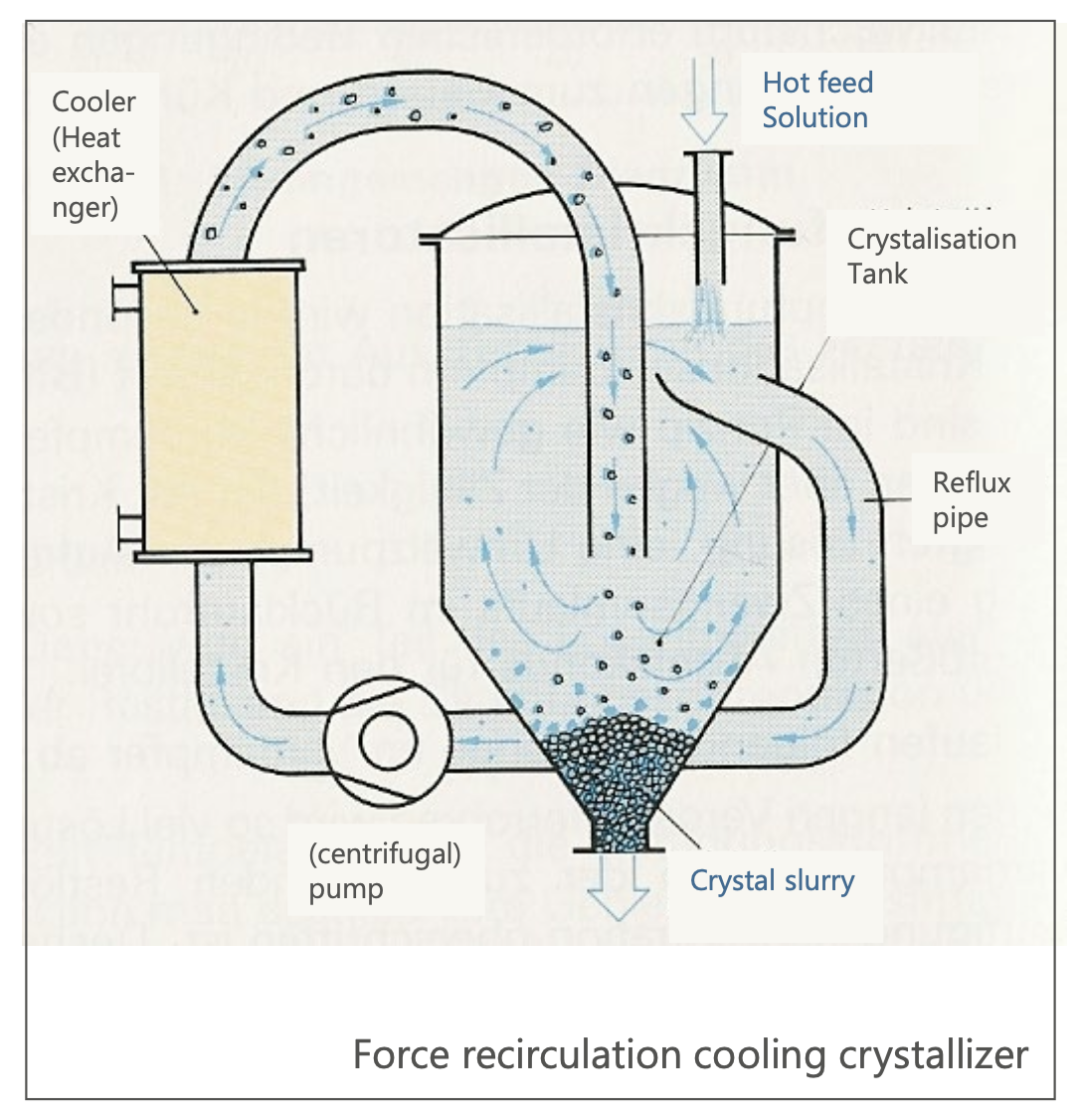
Cooling Crystallizers
Recycling Cooling Crystallizer:
n this type of crystallizer, the solution is circulated through a heat exchanger (cooler) to achieve supersaturation and induce crystallization.
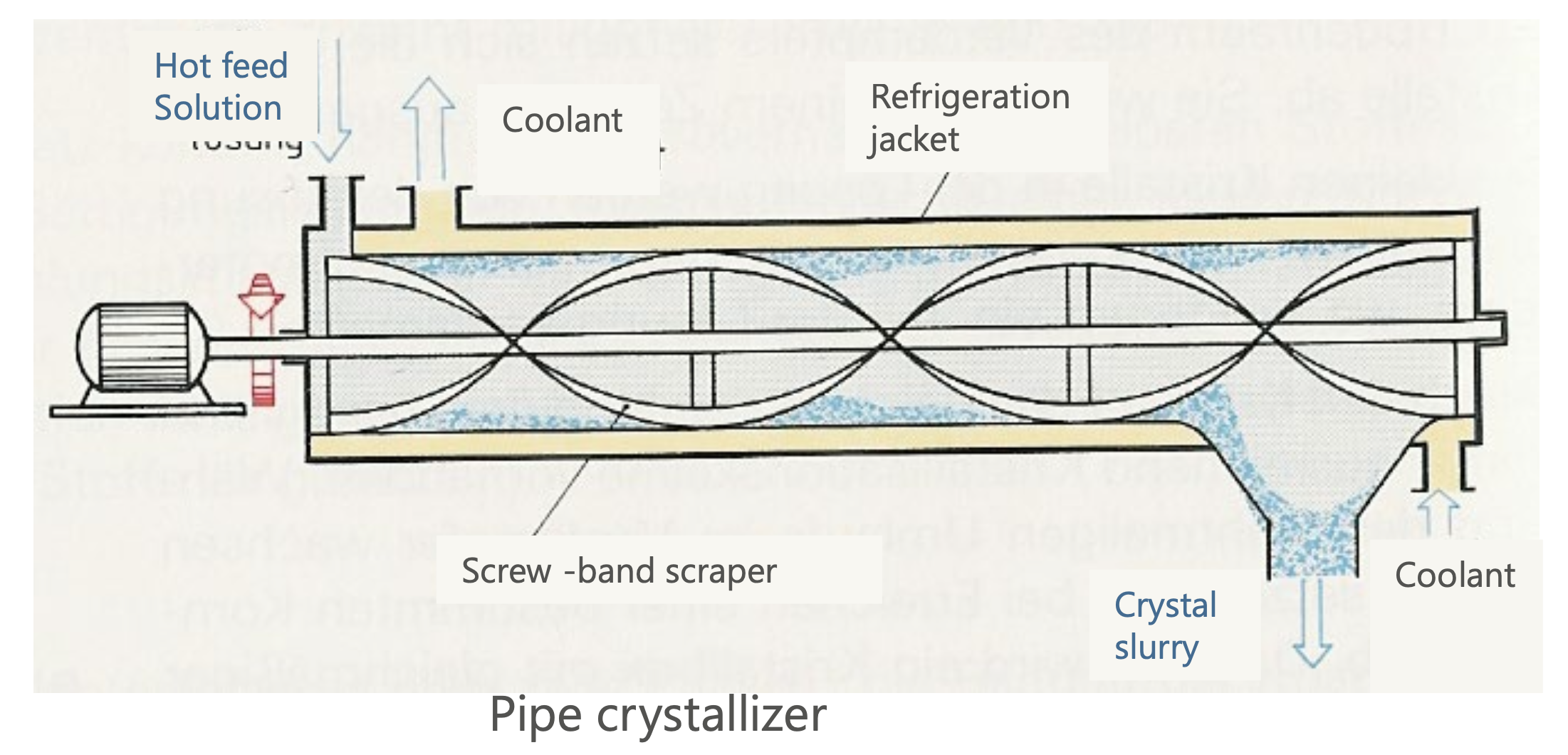
Pipe Crystallizer:
Solution flows through a pipe with a cooling jacket.
Crystals form along the walls and are scraped off.
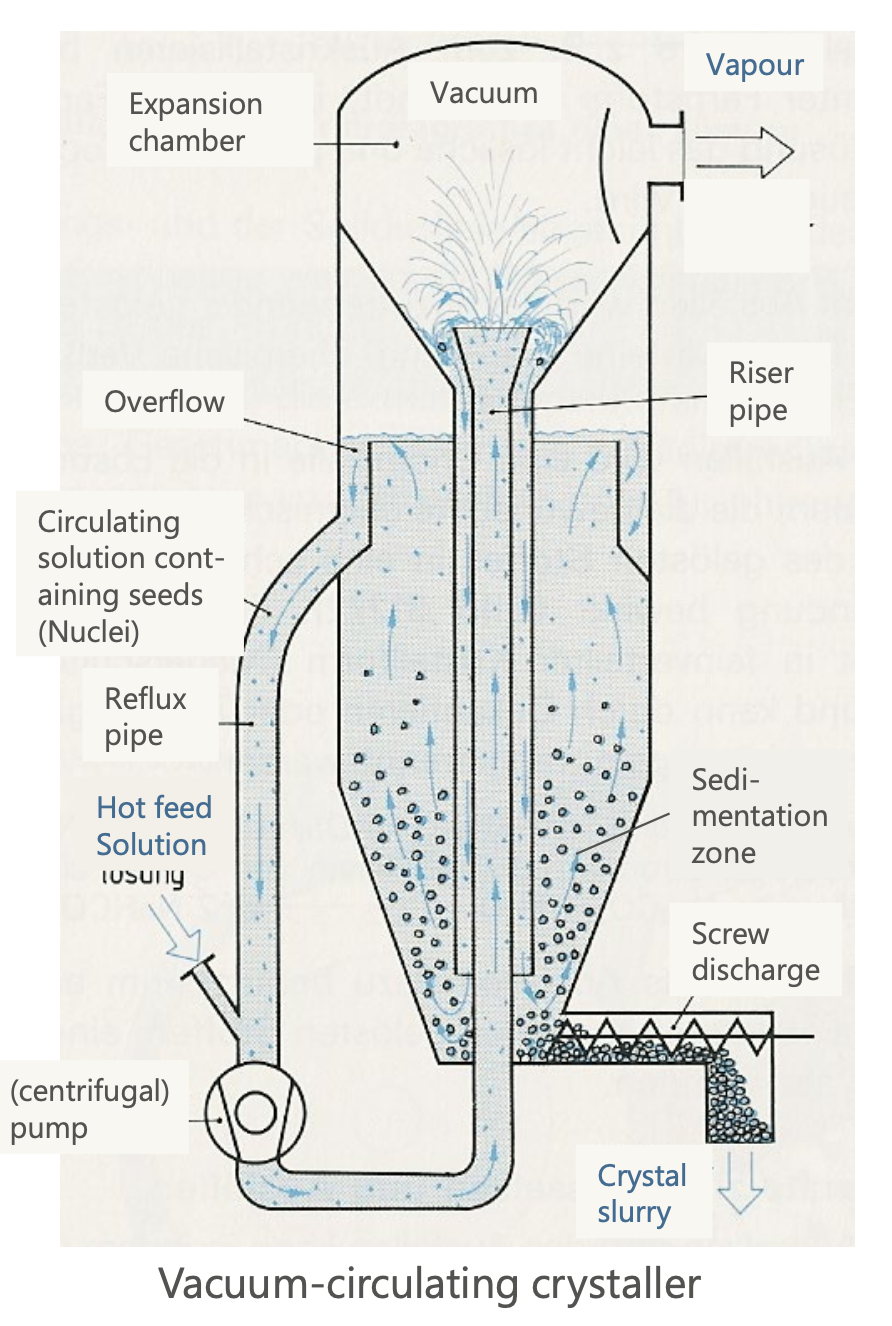
Vacuum Crystallizers
Vacuum Recycling Crystallizer:
Operates under reduced pressure to facilitate simultaneous cooling and evaporation.
At the expansion chamber on top, the pressure drops, the boiling point decreases, and the solution evaporates. This leads to a cooling effect.
The tiny droplets increase the surface area available for evaporation, speeding up the solvent removal process supersaturation point occurs.
In the sedimentation zone, the crystals are collected.
Includes recirculation for crystal growth and extraction.
Vacuum Agitator Crystallizer:
Similar to recycling but with mechanical agitation for enhanced mixing.
Working Principle and Applications of Scraped Surface Heat Exchanger
As soon as the mixture comes in contact with the cold surface, crystals are formed. The blades are scraping the ice off to prevent the formation of the stagnant layer, so the heat transfer would be efficient.
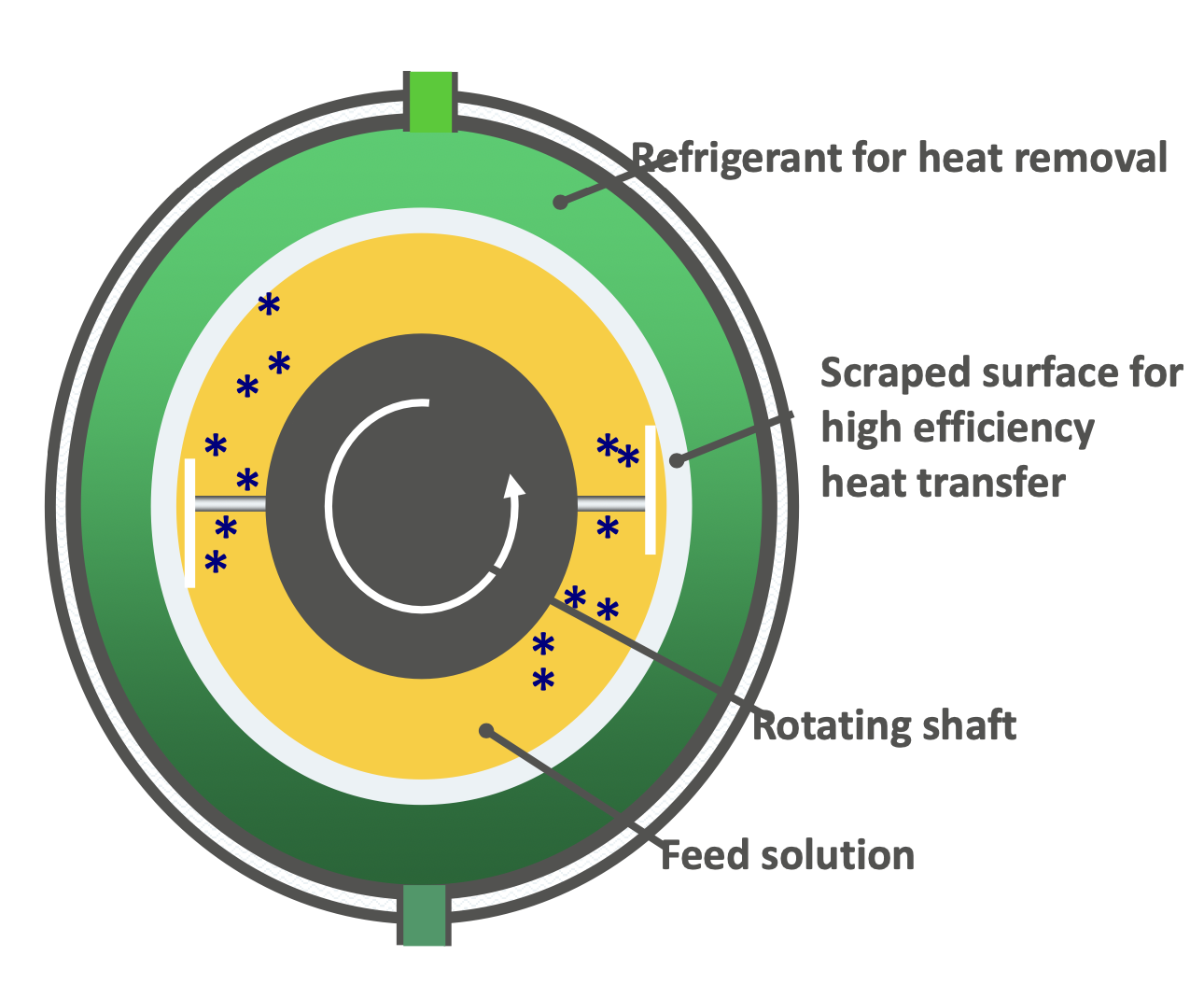
Applications:
Used in ice cream production, chocolate seed crystallization, and margarine production.
Viscosity and Freezing Point Changes with Concentration
With the increase in solute’s concentration, the viscosity increases, and the freezing point decreases, because an extra solute disrupts the freezing point.
Effect of Oversaturation on Crystal Number and Size
High Oversaturation:
Results in many small crystals due to rapid nucleation. Agitation would also increase the number of nuclei, so more crystals can be formed.
Low Oversaturation:
Produces fewer but larger crystals due to slower nucleation and more crystal growth.
Crystallization Kinetics Equation

Mass Transfer Steps During Crystal Growth
Solutes are transferred and incorporated into the crystal’s latice
Ions from the bulk of the solution go through the diffusion boundary layer of the crystal. It’s not the crystal yet, but the concentration gradient layer.
Diffusion of the crystals into the adsorption layer. There, solutes move until they reach the crystal surface that they can attach to.
After the crystal’s surface, solvated (with solvent) and unsolvated particles diffuse along the surface to find an energetically suitable site.
Partial or Total Desolvation of Ions: removal of the solvent before turning into a crystal.
Integration of Ions Into the Lattice
Water which was released during the desolvation step should go back to the bulk solution through the adsroption and boundary layers.
Properties that crystalization depends on
Properties of the solute and solvent.
Degree of oversaturation.
Temperature.
Viscosity of the solution.
Agitation intensity