Fungi/Fungal Diseases
1/101
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
102 Terms
Mycoses
Diseases caused by fungi
Typical manifestation of fungal infections
subacute or chronic infections that commonly relapse over time
Acute fungal disease is
RARE
Cytoskeleton of fungi
has actin microfilaments and tubulin containing microtubules
Ergosterol
A key component of fungal cell wall, similar to cholesterol in animal cells.
How do many antifungal agents work?
by disrupting ergosterol by binding to it and “punching holes” in the cell wall
Complex polysaccharides found in fungal cell walls
mannans
glucans
chitins
ONLY fungus with a capsule
Cryptococcus
Yeast
unicellular
reproduces by budding
if buds do not separate → pseudohyphae
Hyphae
Threadlike, branching, cylindrical tubules composed of fungal cells attached end to end
Molds (Mycelia)
Multicellular colonies composed of clumps of intertwined branching hyphae
Spores
Reproducing bodies of molds
Dimorphic fungi
Fungi that can grow as either a yeast or mold, depending on environmental conditions
most fungi grow as year at body temp
Saprophytes
Fungi that live in and untilize organic matter (soil, rotten vegetation) as an energy source
Fungal dissemination infection are most often acquired by
inhalation of conidia
Local fungal infections are most often acquired by
injection (think thorn) past the skin/mucosal membrane
Adherence
pathogenic factor of fungi
Yeasts especially can colonize the GI tract and female genital tract
Candida best known to adhere to epithelial cells
Mannoprotein components extend from the cell wall are adhesins, and interact with host cell fibronectin and others of the extracellular matrix
Adhesions
mannoprotein components of fungal cell walls that promote attachment of fungi to host tissues, facilitating colonization and infection.
Proteases/elastases
pathogenic factors of fungi
extracellular enzymes that help promote invasion by breaking down host tissues and evading immune responses.
Most injury during fungal infections is due to
the host's immune response, which can cause tissue damage and inflammation.
Healthy people have
innate immunity to most fungal infections, esp opportunistic molds
How is fungal infection most often cleared from healthy human hosts?
through a combination of the innate activity of neutrophils and through the development of an adaptive, TH1-mediated immune response.
How does humoral immunity effect fungal infections
it doesn’t
Antibodies may not corelate with resistance for some fungi
Example: High titers of Coccidioides immitis specific antibodies are associated with
dissemination and a worsening clinical course
however,
Opsonizing antibody is effective for some yeast (Like Cryptococcus and antibodies against its capsule
How does cellular immunity effect fungal infections?
Very helpful!
Systemic disease with deficiencies of neutrophils and TH1 immunity increase
risk for infection, ex:AIDS
chronic steroid treatment
hematologic malignancies
transplant patients
Fungal Diagnosis
Direct examination
KOH
Culture
PCR
Antigen and Antibody detection
KOH
digests tissues but not fungal walls, allowing for the observation of hyphae under light microscrope with or w/out staining
Sabouraud’s Agar
Ideal agar for culturing fungi
Coccidioides diagnosis
Serum antibodies
Cryptococcus diagnosis
Serum and CSF antigen test
Histoplasma diagnosis
Urine antigen
Superficial fungal infections
Pityriasis versicolor
Tinea nigra
Cutaneous fungal infections
Dermatophytosis
infection of the skin
tinea corporis
tines cruris
tinea pedis
infection of the hair : tinea capitits
infection of the nail : tinea ungulum
Candidasis of the skin
the ONLY subcutaneous fungal infection
Sporotrichosis
Opportunisitic fungal infection
Candida
Aspergillus
Zygomycetes
Pneumocystis
Systemic fungal infections
Cryptococcus
Histoplasmosis
Blastomycosis
Coccidioidomycosis
Paracoccidiomycosis
Malassezia Fur fur
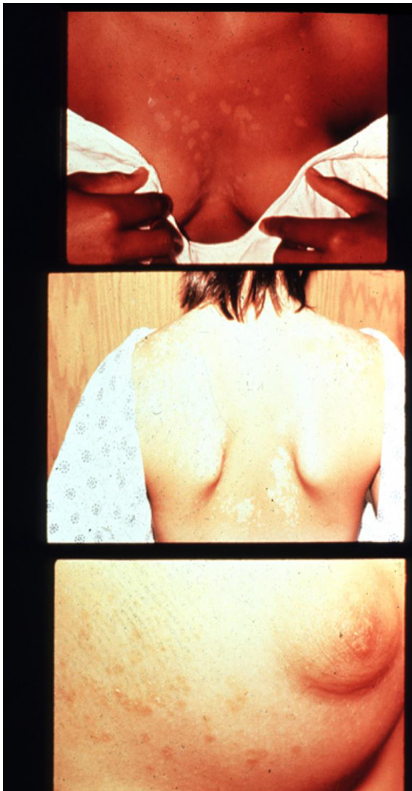
causes pityriasis (tinea) versicolor
pityriasis (tinea) versicolor
Caused by Malassezia fur fur
grows in yeast form in culture media enriched with lipids
sports seasons
Tx selenium sulfide shampoo
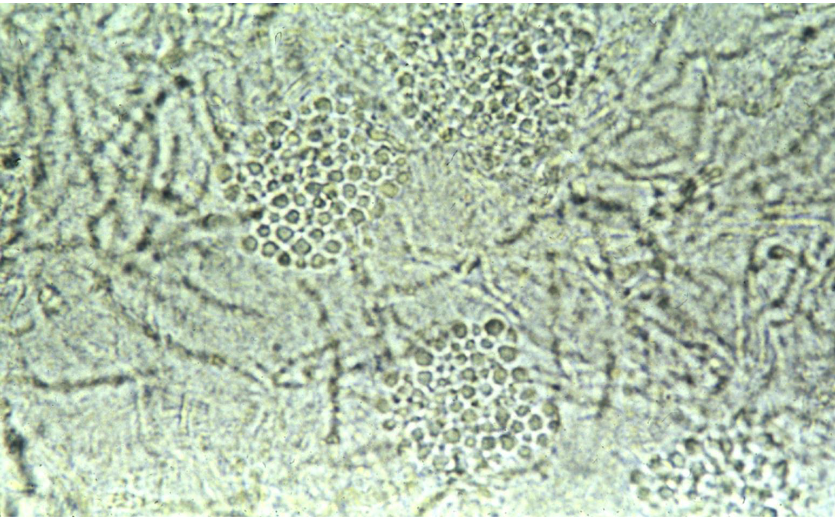
KOH scraping of tinea versicolor
“spaghetti and meatballs”
Tinea nigra
Brown to black lesions usually on
the palms or solesCaused by a melanized, black- pigmented fungi, Hortaea werneckii
It is likely that most patients become infected in aqueous environments (rivers, lakes, and marine areas)
Florida, North Carolina, and
South Carolina
Hortaea werneckii
Causes tinea nigra
Dermatophytes are caused by what three genera?
Microsporum
Trichophyton
Epidermophyton
Dermatophytes clinical presentation
slow growing eruptions of the skin
unsightly but NOT painful
balance between fungal growth and skin desquamation determines who “wins”
self limited but some can become chronic
Dermatophytes Transmission
Close contact with an infected person or animal (yep…the cat!)
Exposure to detached skin scales or hair containing the organism (fomites)
may also result in infectionLocker room floors, barbershops, hotel carpets, movie theater/airplane seats
Onchomycosis
Fungal infection of the nails
Trichophyton rubrum
Fungi that can cause widespread dermatophyte infection, esp if T-lymphocyte cells are deficient
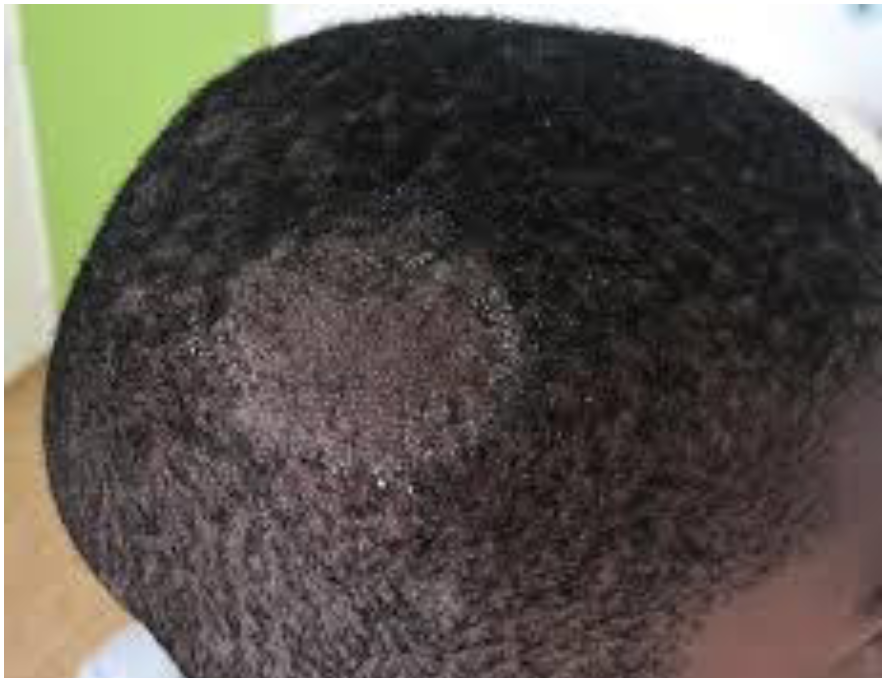
Tinea Captitis
“ringworm of the scalp”
Physical findings can be impressive with a huge inflammatory response
Commonly you can see a “kerion” form
Dx: Scrape the leading edge and do KOH mount and look for hyphae
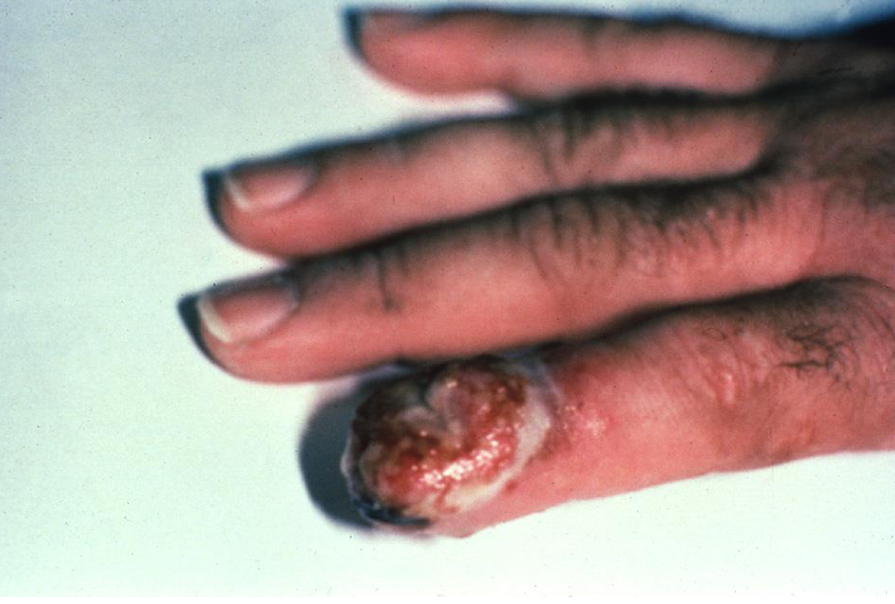
Sporothrix schenckii
Dimorphic
cigar shaped yeast
Found in hay, moss, potting soil, etc
transmission: inoculation through the skin, often the finger
Occupations to look out for regarding Sporothrix schenckii
farmers, gardeners, landscapers
Clinical presentation of Sporothrix schenckii
Development of a nodular lesion that ulcerates
Multiple subcutaneous nodules appear along lymphatic course, these then suppurate and drain
can progress to joint involvement if not treated properly!
General characteristics of opportunistic fungal infections
Grow in multiple morphologic forms, most often budding yeast
Can form hyphae-like structures (germ tubes)
Can do this in the presence of serum (which generally only Candida albicans does)
Germ-tube negative strains may be further identified biochemically as reported as “yeast
not C. albicans”
Other elongated forms with restrictions at regular intervals are called pseudohyphae because they lack the parallel walls and septation of true
hyphae.
Candida Albicans
infection Occurs commonly in the oropharyngeal, gastrointestinal, and female genital tract
Risk factors
Indwelling IV catheters
Indwelling urinary catheters
Prolonged use of antibiotics
Pathogenesis/Immunity of Candida Albicans
One of the most important aspects of its ability to cause pathology is the ability to form biofilms
The biofilms adhere to surfaces and inhibit immune cell function and antifungal
penetration
Antibiotics and immunosuppression increase risk markedly
T-lymphocytes are very important (thus in AIDS, etc. a big problem)
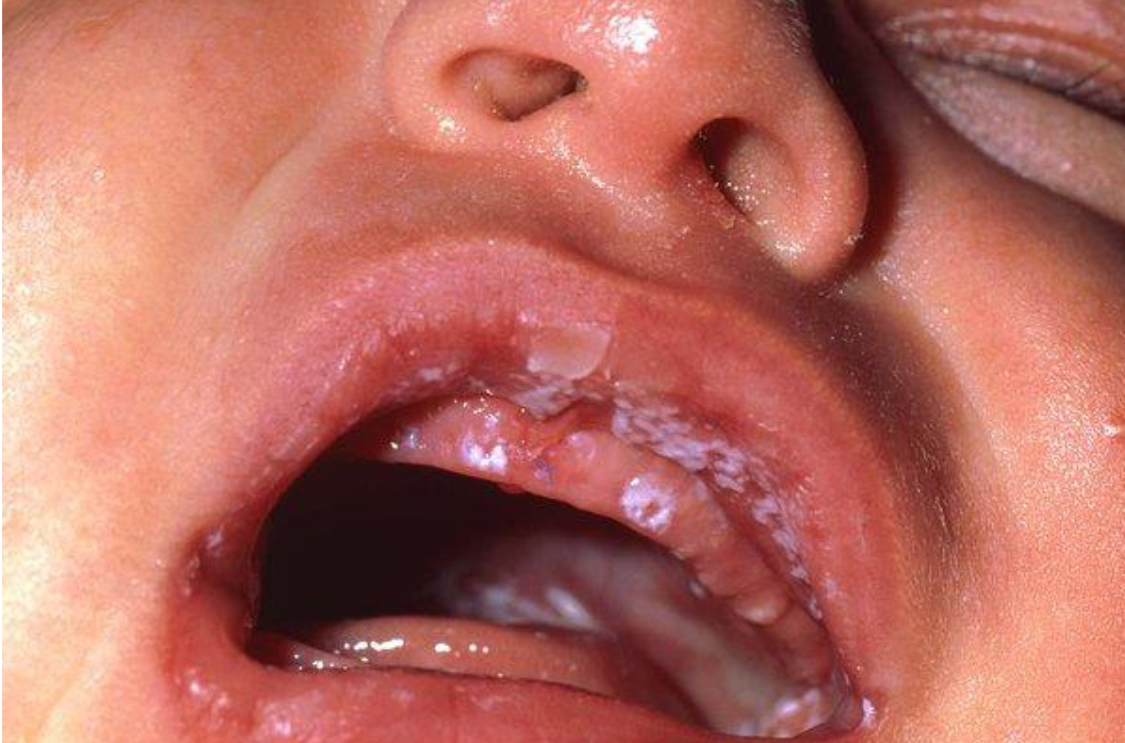
Thrush
Oral manifestation of C. Albicans
Scrapping the fungal plaque with a tongue blade will show underlying mucosal invasion and inflammation
Vulvovaginal Candidasis
Clinical manifestation of C. Albicans
presents with thick, curd-like vaginal discharge and
itchingFound in Skin folds and other moist areas in the groin, under the breast, DIAPER AREA!
C. Albicans Esophagus and upper GIT lesions
Clinical manifestation of C. Albicans
most common in AIDS and immunocomp patients
presents as painful swallowing or substernal chest pain
C. Albicans in the uninary tract can cause
Cystitis, pyelonephritis
Most commonly in hospitalized patients or those with urinary catheters
C. Albicans in Disseminated disease
Very high mortality
Seen in Hospitalized patients
burns
IV caths
GI alterations
Colonized prosthetic devices with candida
Endophthalmitis with Candida
ALWAYS check for this in those with Candidemia, as it is very common!
White cotton ball expanding on the retina or floating free in vitreous humor
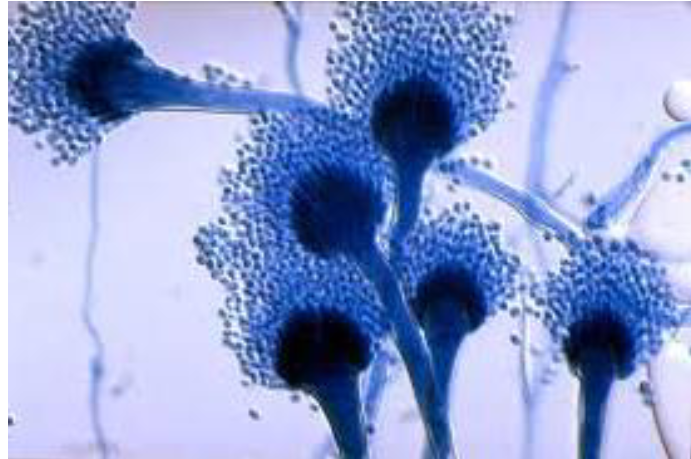
Aspergillus
Rapidly growing
Seen most often in patients with severe immunocompromising positions
In culture and during infection grows as branching septate hyphae
Picture shows its asexual, conidium-forming structure, which is unique to this organism
Apsergillus epidemiology and pathogenesis
infection Most commonly caused by inhalation
Once inhaled, this species is small enough to reach the terminal airways and alveoli
RARE for healthy individuals to get infection to to innate immune cell protection
Host factors that increase risk of Aspergillus infection
Bronchiectasis
Severe emphysema
Neutropenia
Lung transplantation
First line of defense against Aspergillus
Pulmonarly alveolar macrophages, which phagocytose and kill this fungus prior to germination
Which fungal infection do AIDS parients rarely get, suggesting T-cell mediated immunity is less important than innate immunity in preventing infection
Aspergillus
4 main ways Aspergillus affects human hosts
Aspergillus pneumonia
Disseminated aspergillosis
Allergic respiratory disease
Aspergilloma (fungus ball)
Aspergillus Pneumonia
Lung is primary organ involved
In normal humans, immune responses are usually able to clear spore before infection
In those with anatomic defects: Emphysema and bronchiectasis (cystic fibrosis) have regions within their lungs that offer protected sites for fungal germination and growth
“smoldering” infection can result in symptoms of chronic bronchopneumonia with flares of cough
and worsening respiratory function
In those with immune defects: Develop a progressive and immediately life-threatening pneumonia without necessary anatomic problems
Disseminated Aspergillosis
After a primary infection in immunocomp patient, fungus disseminates into to the bloodstream to go anywhere in the body
Scariest site to spread: Central nervous system
High mortality
Allergic Respiratory Disease
In patients, with allergic tendencies, the accumulation of excess mucus in the areas may provide a growth substrate for inhaled Aspergillius
However, as potent allergens, the fungi may induce a cycle of progressive
inflammation creating mucinous substrate for fungal growth.May grow in the sinuses and larger airways and cause chronic allergic symptoms
Children with asthma are particularly prone to a condition called allergic bronchopulmonary aspergillosis (ABPA)
Aspergilloma (fungus ball)
Patients with prior lung infections can develop pulmonary scarring and cavities
Fungal spores could grow in these cavities and make macroscopic fungal colonies
Can invade but instead induce mechanical trauma
They literally roll around in the cavity!
Cause hemoptysis but if near large blood vessels can cause life-threatening bleeding
Aspergillus Diagnosis
Can be found in cultures of infected tissues, BUT
The problem is distinguishing colonization with true invasive disease
Bronchoalveolar lavage (BAL) or lung biopsy is commonly required
Aspergillus serology can be helpful in ABPA but not invasive disease
Zygomycetes (Mucormycosis)
Highest risk of infection in:
Neutropenia
DM
Immunosuppresed pts on corticosteroid therapy
Inhalation is most common route of infection
Best known for causing rhinocerebral mucormycosis
Rhinocerebral Mucormycosis
When Zygomycetes (Mucormycosis) Penetrates the sinuses, palate and invade to the base of the brain!
Classic is diabetic with a bloody nose or nasal stuffiness on one nares and when you look in
there you see a black necrotic areaTx: Extensive surgical debridement with high dose antifungal therapy
Pneumocystis jirovecii
Common colonizer of the human airway
Lethal in AIDS patients with <200 CD4+ cell counts
75% are infected by age 4, but Alveolar macrophages clear the infection
In healthy ppl, CD4 cells cause host inflammatory response by recruiting additional immune cells (monocytes, macrophages), which eliminate the organism
Clinical Presentation of Pneumocystis jirovecii
AIDS pts w/ < 200 CD4 cell counts
Dyspnea on exertion
Elevated plasma levels of 1-3-beta-D-glucan (component of cell wall)
On high resolution computed tomography (НRСТ), ΡJР pneumonia typically manifests as bilateral patchy or nodular ground glass opacities
Diagnosis of Pneumocystis jirovecii
Induced sputum or bronchoalveolar lavage (BAL) with DFA or PCR or Gomori-methenamine silver stains
If beta-D-glucan test is available, it can help distinguish colonization versus true infection in patients with atypical clinical presentations.
Treatment of Pneumocystis jirovecii
TMP/SMX for 21 days
Prophylaxis for those with AIDS and CD4+ < 200 cells
Cryptococcus neoformans
life cycle involes asexual and sexual forms
Identitifcation involves the identification of the enzyme phenol oxidase, which is solely produced by this fungus
Histo identification of Cryptococcus
methenamine silver stain
Mucicarmine stain highlights both the yeast form and the
capsule and is specific for this fungusFontana-Masson stain reveals melanin
contained in the yeast
Cryptococcus capsule
Visualized using india ink
has antiphagocytic properties and is an important virulence determinant
Phenol oxidase
unique to C. Neoformans
catalyzes one step in the conversion of phenolic compounds to melanin
Most patients with cryptococcus are
Immunocompromised
AIDS
Cryptococcus in AIDS patients
Disseminated infection is a serious opportunistic infection that occurs in patients with AIDS with CD4+ count < 100 cells/microL
meningitis is the most frequently encountered manifestation among those with
advanced immunosuppressionSymptoms of cryptococcal meningoencephalitis typically begin indolently over a period of 1-2 weeks.
The most common symptoms are fever, malaise, and headache
“worst HA of their life”
~6% present with focal neurologic deficits, such as CN palsies.
Others present with tachypnea and skin lesions resembling molluscum contagiosum.
Dx of Cryptococcus
MRI or CT scan of the Head is done first to make sure not increased intracranial pressures
Spinal tap (lumbar puncture, LP) is mandatory to make the diagnosis
Histoplasma Capsulatum
Themrally dimorphic fungus
This is how it gains entry into humans via inhalation of this environmental spores
can become chronic or disseminated in some competent and commonly in immunocompromised (AIDS)
Risk Factors
Chicken coops or farm buildings with large accumulations of bird droppings, bird roost sites, caves
Excavation/construction/demolition/remodeling
Macrophages initially injest but do not kill what fungus?
Histoplasma capsulatum
Time frame of cellular immunity to Histoplasma Capsulatum
10-14 days after exposure
Symptoms of Histoplasma Capsulatum
2-4 weeks after exposure develop Fever, chills, headache, myalgias, anorexia, cough, and chest pain
Chest pain is substernal and often is aggravated by deep inspiration; patients may experience pleuritic pain
Coryza and sore throat are NOT typical and should suggest alternative diagnoses.
CXR of histoplasma capsulatum
Enlarged hilar or mediastinal lymph nodes with focal infiltrates but may be normal.
Calcification of mediastinal nodes or lung lesions are seen within a few months in children, but calcification usually takes several years to develop in adults.
Blastomyces Dermatitidis
Has thermal dimorphism
mold at room temp
yeast at body temp
due to hybrid histidine kinase
Transmission:
Inhalation of conidia
PMNs can ingest conidia and kill 50%
In contrast, yeast forms are more resistant to phagocytosis and killing.
PMNs can only kill 20% of yeast, which are generally too large for ingestion
Conidia, the infectious stage, are converted to the yeast phase in tissue.
This conversion results in a survival advantage , which contributes to infection
Virulence Factor of Blastomyces Dermatitidis
Conversion to the yeast form induces the expression of an essential virulence factor, BAD-1.
BAD-1 is expressed on the cell surface and is released into the extracellular matrix.
BAD-1 functions as an adhesin that can bind to CR3 and CD14 of macrophages
BAD-1
Essential virulence factor of B. Dermatitidis
Host defense of B. dermatitidis
The major acquired host defense is cellular immunity
Mediated by antigen-specific T lymphocytes and lymphokine-activated macrophages.
Antibody production appears not to provide protection
B. Dermatitidis outbreaks/epidemics are often associated with
Waterways
Highway construction near waterways
Paper Mill
Beaver dams (bulldozing them and Scouts deconstructing them)
blastomyces dermatidis isolated infection risk
Pet dogs who have pneumonia and die or have known blastomycosis!
Clincal Manifestation of Blastomycosis
Asymptomatic infection (50%)
Acute pneumonia
Chronic pneumonia
Extrapulmonary disease
3-6 week incubation period for pulmonary disease
Extrapulmonary symptoms have variable incubation periods
Reactivation (both with pulmonary and extrapulmonary
symptoms) can occur in immunocompromised (taking TNF-alpha inhibitors or steroids)
Sites of infection of Blastomycosis
Pulmonary - 91%
Skin - 18%
17% had 2 or more sites of infection
Dx of Blastomycosis
Urine antigen and try to get sputum or lung specimens for direct microscopy, culture, and histopathology
Coccidiodes immitis
Infection is usually acquired by inhalation of arthroconidia
The cellular immune response is important
Causes pulmonary lesions that are characterized by caseating granulomata with an active T-cell response
Coccidioidomycosis—Disease Manifestations
Asymptomatic infection
Dx is usually a part of screening due to immunocompromised or pulmonary disease evaluation
Primary Coccidioides Pneumonia (AKA Valley Fever)
Extrathoracic Nonmeningeal Disease
Meningitis
Primary Coccidioides Pneumonia (AKA Valley Fever)
Incubation: 7-21 days after an exposure
Localized pneumonia
Rheumatologic → arthralgias
Skin → erythema nodosum
Dx: IgM and IgG serologic EIA tests
Immunodiffusion tests are then performed to confirm initial EIA positivity
Tx: Fluconazole
Risk of Reactivation if become immunocompromised in the future
Extrathoracic Nonmeningeal Disease
Clincal manifestation of Coccidioidomycosis
Lesions become evident within weeks to months after initial exposure
Skin or subcutaneous soft tissue
Osteoarticular involvement
Vertebral infection
Tx: Fluconazole/other meds with surgical debridement