Orgo chem quiz 3
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Chair conformation
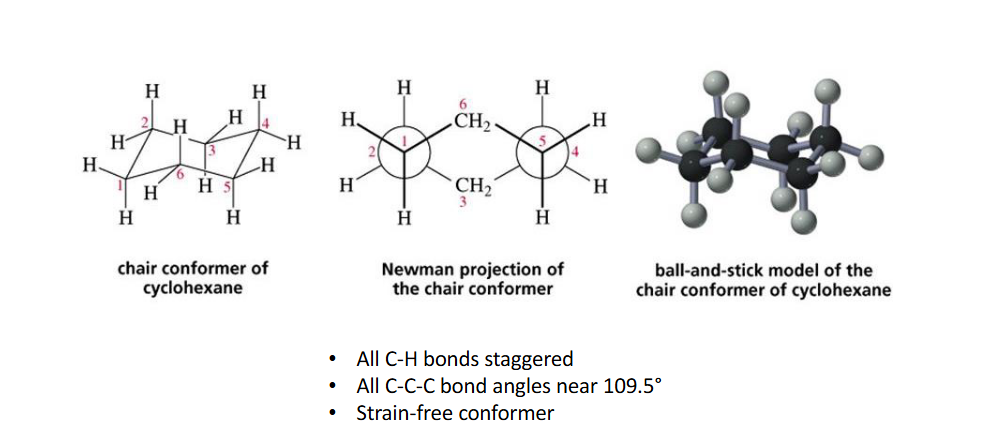
Axial bonds
(perpendicular to the plane of the ring)
-straight up or straight down
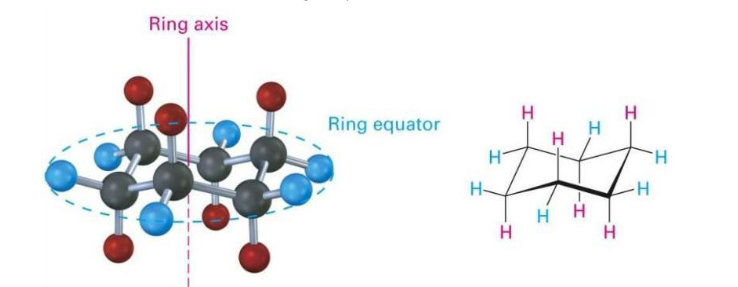
Equatorial bonds
around the ring equator
-slightly down or slightly up
Ring flipped conformer
Axial substituents (or atoms) become equatorial in ring-flipped conformer.
Equatorial substituents (or atoms) become axial in ring-flipped conformer.
-Ring flip does not change cis/trans relationship of substituents.
-But it does not change stereochemistry — the “up” stays up, and the “down” stays down.
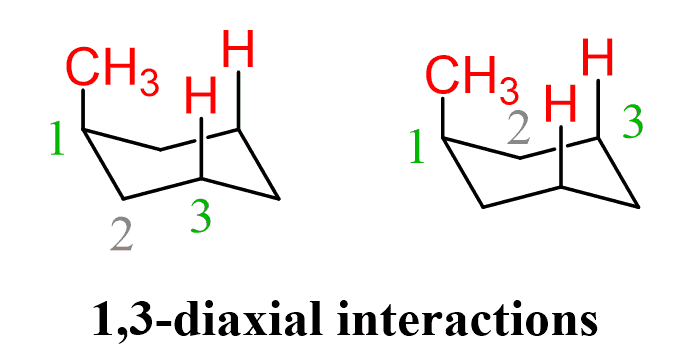
1,3-diaxial interactions
-unfavorable, steric interactions
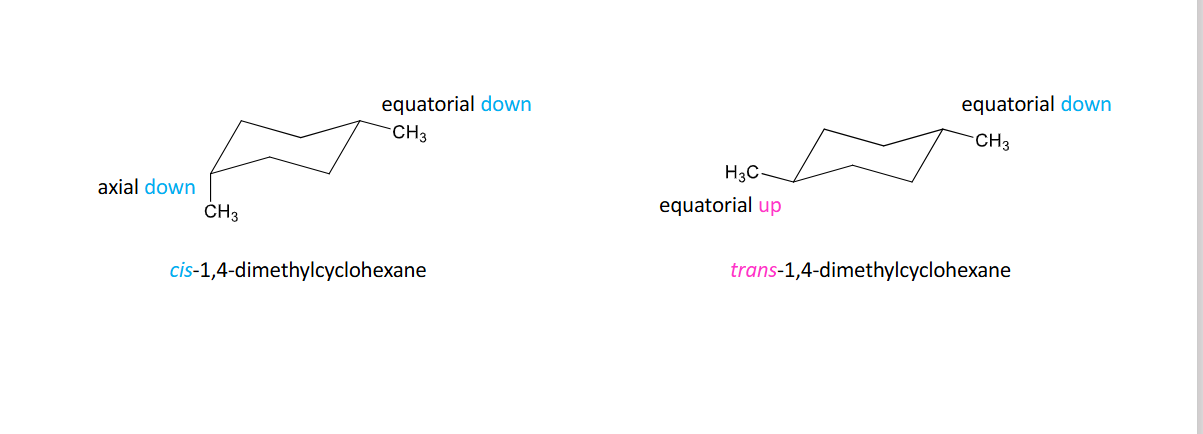
Disubstituted Cyclohexanes
To draw most stable conformer:
• number your chair
• draw largest substituent in equatorial bond and determine if it has up or down orientation
• draw second substituent according to cis or trans relationship to first substituent drawn
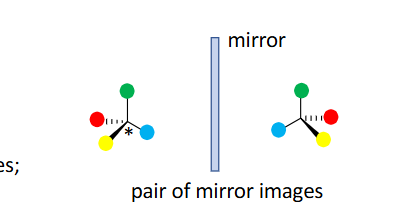
enantiomers
mirror images are NOT superimposable
-have same physical properties (e.g. boiling point) and
chemical properties.
-have different biological activity (important implications in
biological systems and pharmacology)
-You invert every single chiral center — meaning all wedges become dashes and vice versa. → This gives you the mirror image.
For chair conformations:
To draw the enantiomer: invert both chiral centers
Flip Br from axial up → equatorial down
Flip CH₃ from equatorial down → axial up

Chiral molecules
have “right-handed” and “left-handed” versions and those are non-superimposable mirror images of each other (enantiomers)
Chiral center
(or stereocenter, or stereogenic center, or asymmetric center) is an sp3-hybridized carbon with 4 different atoms (or groups) bonded to it (marked with asterisk)
-A molecule with n number of stereocenters has 2^n possible stereoisomers.
Cholesterol has 28 = 256 possible stereoisomers!
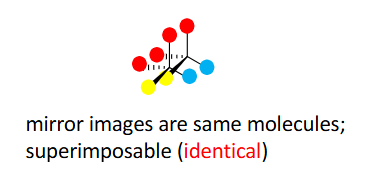
Achiral molecules
have superimposable mirror images (mirror images are the same molecule). Symmetry = no chirality
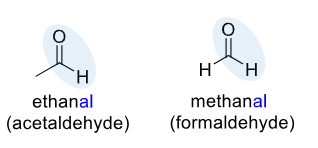
Aldehyde
an organic compound containing a carbonyl functional group (a carbon atom double-bonded to an oxygen atom) with at least one hydrogen atom attached
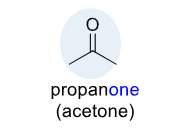
Ketone
consists of a carbonyl group (C=O) bonded to two carbon atoms,
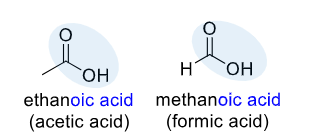
Carboxylic acid
the -COOH functional group, consisting of a carbonyl group (C=O) and a hydroxyl group (-OH) bonded to the same carbon atom
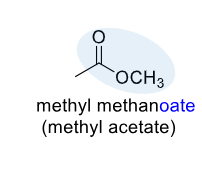
Ester
a functional group in organic chemistry with the general structure R-CO-OR', formed by replacing the hydrogen atom of a carboxylic acid's -OH group with an alkyl or aryl group (R')
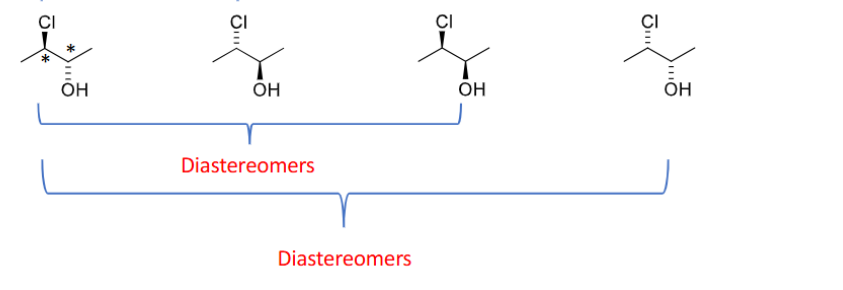
Diastereomers
are stereoisomers that are not mirror images. At least 2 chiral centers must be present
-have different physical and chemical properties.
-You invert only some chiral centers — at least one stays the same.
-cis/trans isomers are a type of diastereomer.
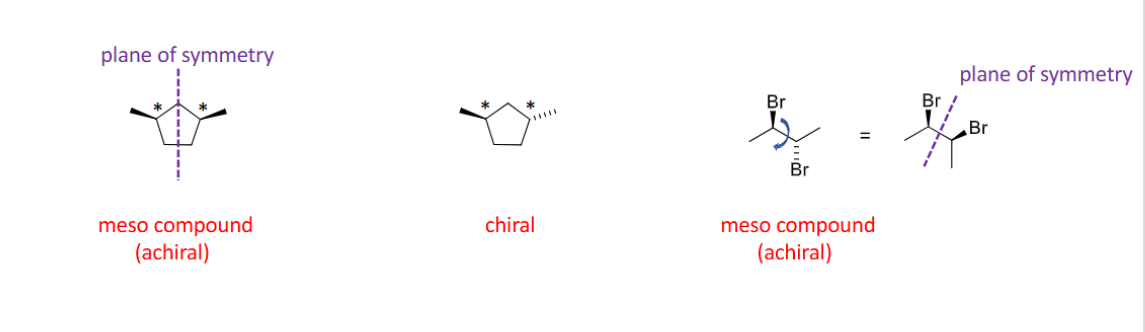
Meso compound
has at least two (or more) chiral centers but also has a plane of symmetry.
- overall achiral
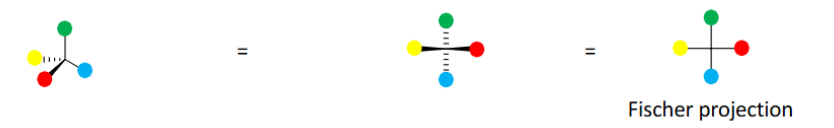
Fischer Projections
Developed by Emil Fischer as a quick way to indicate stereochemistry in chiral molecules with
multiple stereocenters (common in carbohydrates).
Projection of tetrahedral carbon on a flat surface (tetrahedral carbon at every intersection of
two crossed lines)