Biochem 30 | Oxygen Toxicity & Free Radical Injury
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
17 Terms
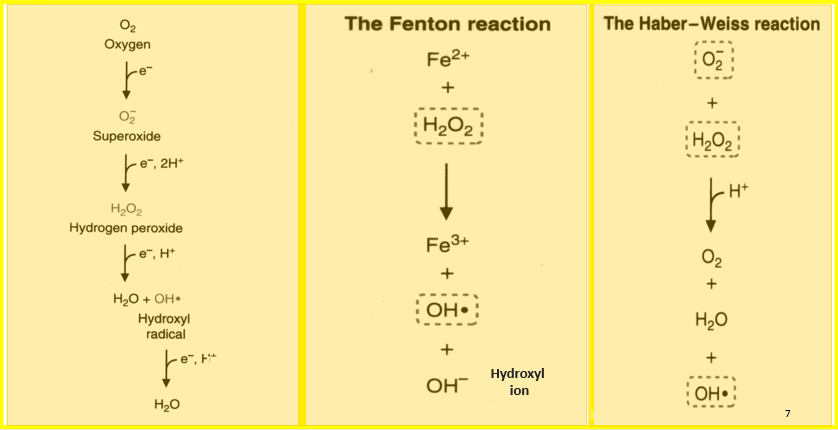
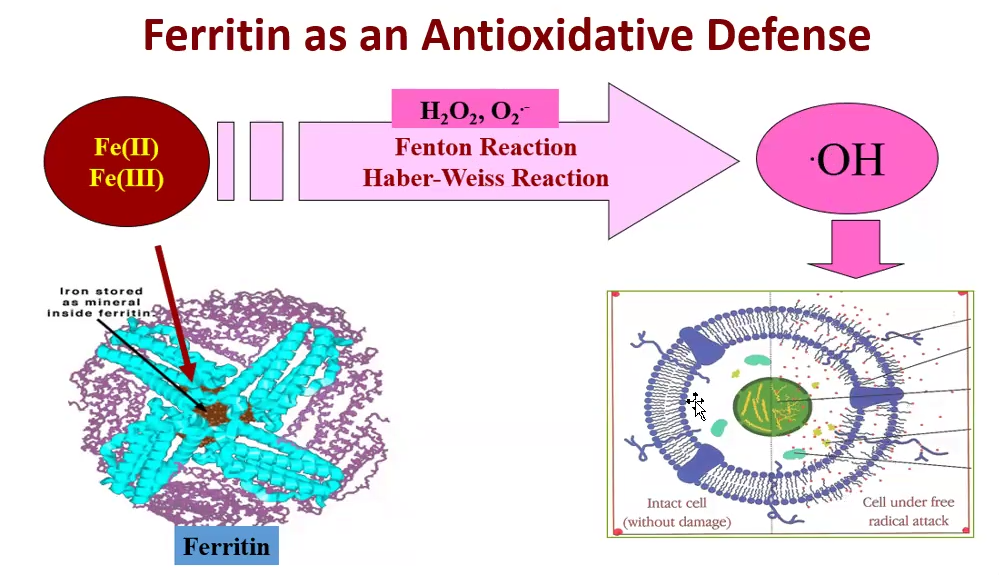
What is the Fenton Reaction?
The Fenton reaction is a chemical process that produces highly reactive hydroxyl radicals (•OH) by reacting hydrogen peroxide (H₂O₂) with iron ions (Fe²⁺).
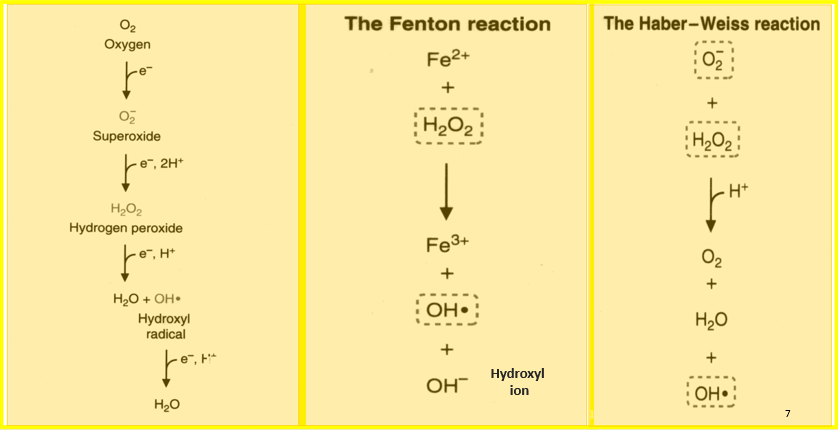
What is the Haber-Weiss Reaction?
The Haber-Weiss reaction is a two-step process where superoxide anion (O₂•⁻) reacts with hydrogen peroxide (H₂O₂) in the presence of iron ions. This reaction produces hydroxyl radicals and other reactive species, contributing to oxidative stress in cells. Similar to the Fenton reaction, it highlights the harmful effects of reactive oxygen species.
How is peroxynitrite formed?
When nitric oxide (NO) reacts with superoxide anions (O₂⁻).
What is the mitochondrial ETC electron leak and what does it produce?
Where electrons escape from the mitochondrial electron transport chain before reaching the final electron acceptor (oxygen), prematurely reacting with oxygen and generating reactive oxygen species (ROS) like superoxide, instead of being used to produce ATP;
Basically, it's a leakage of electrons from the ETC during the process of oxidative phosphorylation, leading to potential cellular damage if excessive.
What is an oxidase enzyme?
An oxidase is an enzyme that catalyzes the transfer of electrons from a substrate to molecular oxygen (O₂), leading to the formation of reactive oxygen species.
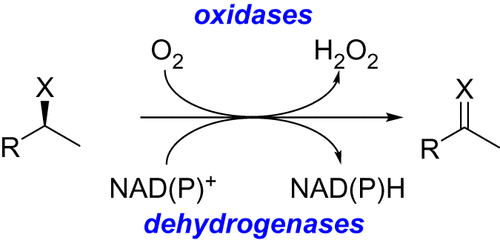
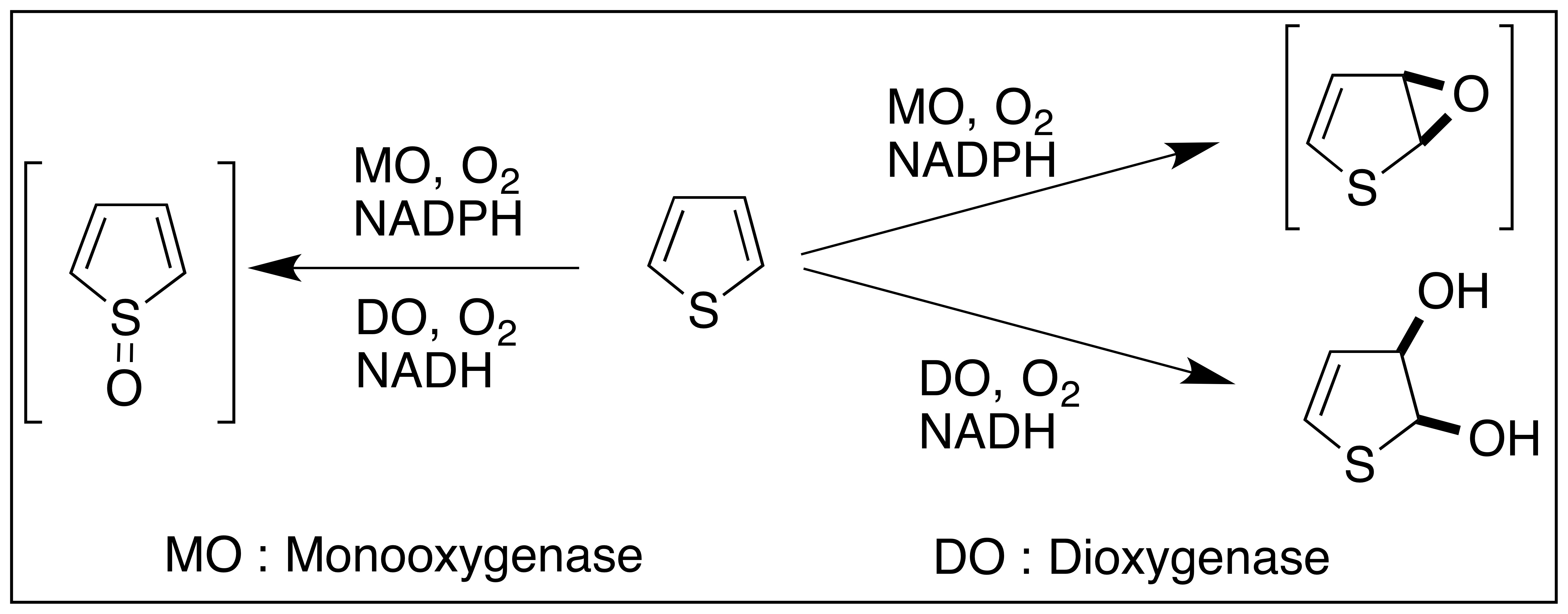
What is the difference between oxidase, monooxygenase and dioxygenase?
Oxygenase: Oxidases are enzymes that transfer electrons from a substrate to oxygen, but they don’t incorporate oxygen into the substrate. (Oxygen (O₂) is reduced to either water (H₂O) or hydrogen peroxide (H₂O₂))
Monooxygenase: Monooxygenases incorporate one atom of oxygen from O₂ into the substrate, and the other oxygen atom is reduced to water. (Ex: CP450 enzymes)
Dioxygenase: Dioxygenases incorporate both oxygen atoms from O₂ directly into the substrate.
What is the fundamental function of the cytochrome P450 enzyme?
The function of P450 enzymes is to break down drugs and toxins by adding an oxygen atom to them. This makes these substances easier for the body to eliminate, mainly through the liver.
What reactive oxygen species are produced during the catalytic mechanism of P450?
Superoxide anion (O₂⁻): This is formed when molecular oxygen (O₂) accepts one electron during the initial steps of the reaction.
Hydrogen peroxide (H₂O₂): This can be produced as a byproduct when two superoxide molecules react.
Hydroxyl radical (•OH): This is formed during subsequent reactions and is a highly reactive species that can cause damage to cellular components.
Oxygen (O₂): In some cases, molecular oxygen is also released as a product after the P450 reaction.
What is oxidative stress?
Oxidative stress occurs when reactive oxygen species are produced in excess and overwhelm the cell's ability to handle them.
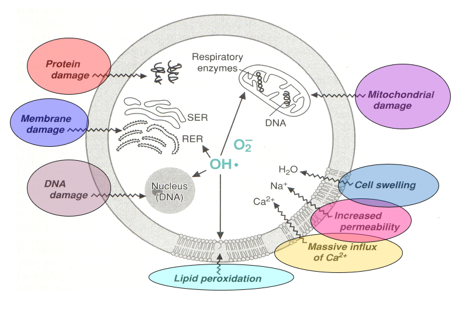
How do reactive oxygen species (ROS) harm cells?
Protein Damage: Proteins can become misfolded or nonfunctional.
Membrane Damage: Cell membranes can lose their integrity, affecting cell function.
DNA Damage: Genetic material can be harmed, leading to mutations.
Lipid Peroxidation: Fats in the cell membrane can break down, which affects membrane structure.
Mitochondrial Damage: Mitochondria may become dysfunctional, reducing energy production.
Cell Swelling: Cells can swell due to changes in their internal environment.
Increased Na+ Permeability: More sodium can enter the cell, disrupting ion balance.
Massive Ca²⁺ Influx: Excess calcium can enter the cell, leading to further damage.
Why Are Unsaturated Fatty Acids Susceptible to Free Radical Attack?
Unsaturated fatty acid chains in the lipid bilayer are vulnerable to free radicals because they have rich sources of electrons that free radicals can react with.
What is the evidence of free-radical damage to membranes?
With the presence of malondialdehyde in blood and urine, which indicates that lipid damage has occurred due to reactive oxygen species.
Takeaway: Malondialdehyde is a biomarker for lipid peroxidation and damage
Why is mitochondrial DNA far more susceptible to ROS damage than nuclear DNA?
Mitochondrial DNA is 10 times more susceptible to ROS damage than nuclear DNA.
It lacks protective histones and higher-level structural organization.
There is a high rate of ROS generation from the mitochondrial electron transport chain (ETC) due to electron leak.
Mitochondrial DNA is located near the inner membrane of mitochondria, where the ETC is situated, making it easier for ROS to attack.
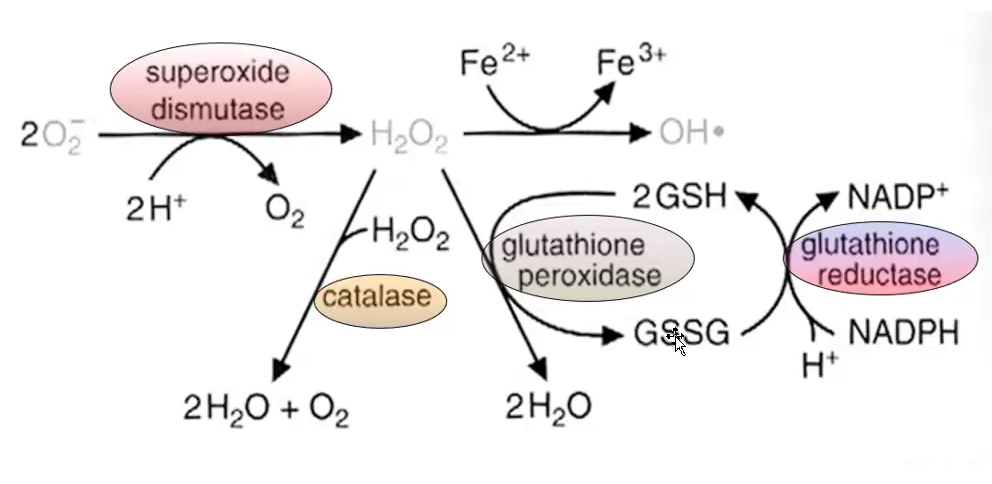
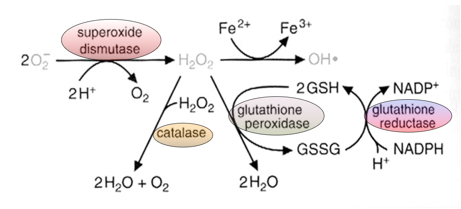
What is the function of SOD and catalase?
Destroys superoxide and H2O2
SOD (Superoxide Dismutase): Converts superoxide (O₂•⁻) into hydrogen peroxide (H₂O₂), helping to reduce oxidative stress.
Catalase: Breaks down hydrogen peroxide (H₂O₂) into water (H₂O) and oxygen (O₂), further protecting cells from ROS damage.
What is the function of vitamin E and why is it found in membranes?
Vitamin E acts as an antioxidant, destroying lipid radicals in membranes to protect cells from oxidative damage. It is found in membranes because it helps preserve the integrity of lipid bilayers.
What is the function of Ferritin?
Ferritin binds free iron to prevent it from interacting with hydrogen peroxide (H₂O₂), reducing the risk of oxidative damage caused by iron-catalyzed reactions.
What are the reactions catalyzed by glutathione peroxidase and glutathione reductase? What is the role of glutathione in these reactions?
Glutathione Peroxidase: Converts hydrogen peroxide (H₂O₂) into water (H₂O) using glutathione, which gets oxidized.
Glutathione Reductase: Converts oxidized glutathione (GSSG) back to reduced glutathione, using NADPH.
Role of Glutathione: Acts as an antioxidant, providing electrons to neutralize ROS and protect cells.