IMFs
1/12
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
13 Terms
INTRAmolecular forces (2)
Forces (bonds) that hold a molecule together. Means "within" the molecule.
INTERmolecular forces (3)
Attractive forces between molecules that allow them to interact with each. Means "between" molecules. Weak (in comparison to intramolecular forces)
Examples of intermolecular bonds (3)
Hydrogen bonding
Dipole-dipole attractions
Electrostatic attractions
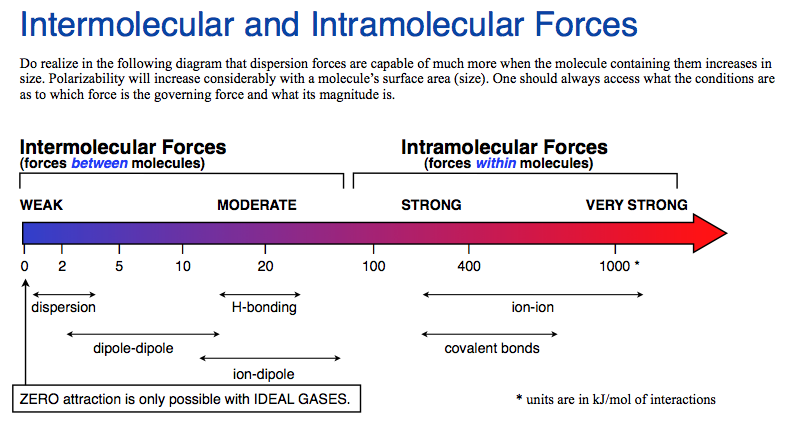
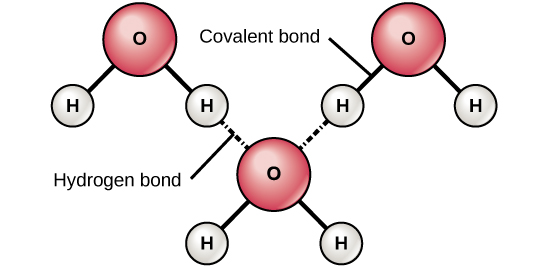
Hydrogen bonding (2)
Attractive force between the hydrogen attached to an electronegative atom of one molecule and an electronegative atom of a different molecule
Occurs in polar molecules
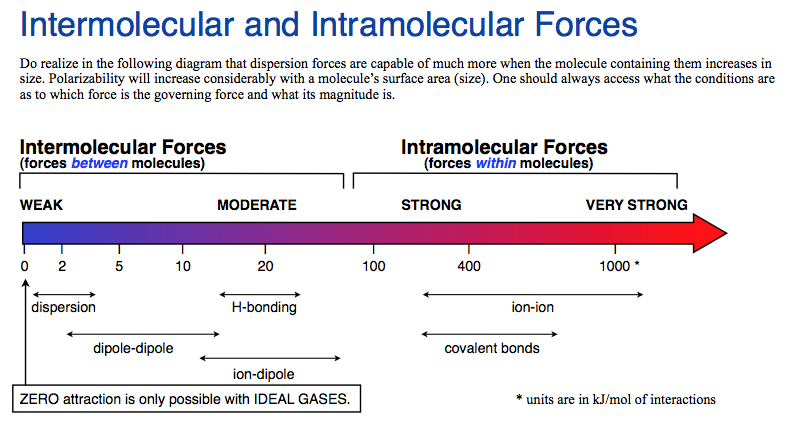
Dipole-dipole (3)
The attraction between the positive end of one molecule and the negative end of another molecule
Occurs between polar molecules
Weaker than hydrogen bonding
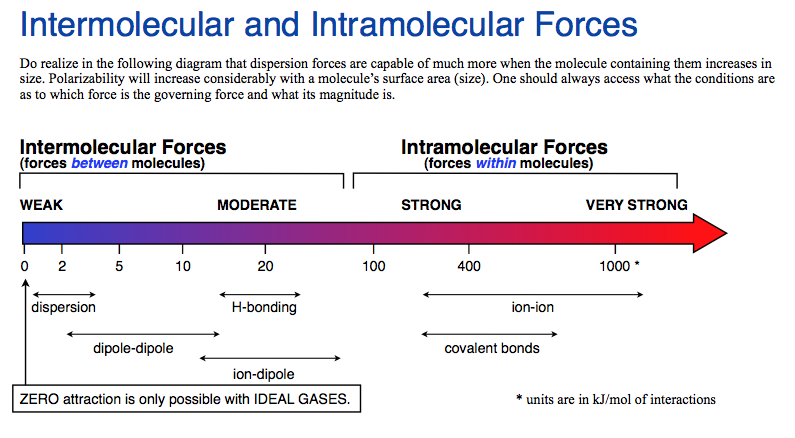
[London] Dispersion forces (3)
Forces that are temporarily created by the attraction of the nucleus to electrons of other molecules
occurs between any type of compounds
Weakest
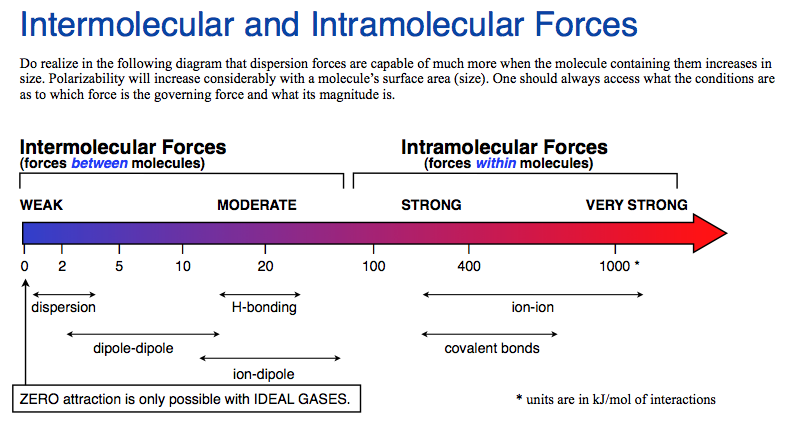
Ion-Dipole force (3)
The attraction between the an ion and the charge of another molecule
• Occurs between an ion and a polar molecule
• Strongest intermolecular force
How IMF affect evaporation? (4)
Stronger IMFs = Slower Evaporation
More energy is needed for a molecule to escape into the gas phase.
Weaker IMFs = Faster Evaporation
Less energy is needed to escape.
How do molecules evaporate & cause a cooling sensation? (3)
Molecules at the surface must absorb enough energy to overcome intermolecular forces (IMF) holding them in the liquid.
Once they break free from these forces, they evaporate into gas.
Since the fastest (highest kinetic energy) molecules escape, the liquid cools down (loses kinetic energy).
How would you expect water to evaporate? (2)
IMF type: Hydrogen bonding (very strong).
Evaporation is slow because it takes a lot of energy for a particle to escape
How would you expect ethanol to evaporate? (5)
IMF type: Hydrogen bonding and some dipole-dipole interactions.
Evaporates at a moderate pace because the H-bonds are quite strong it, but dipole-dipole are weaker than H-bonds
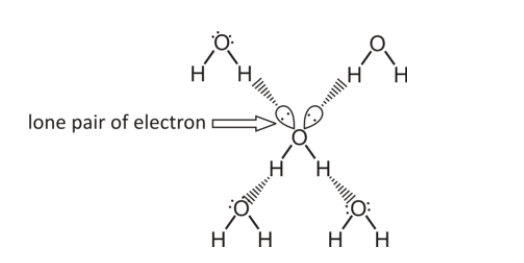
Why are ethanol’s IMF weaker than water? (6)
Each water molecule can form up to 4 hydrogen bonds:
• 2 bonds from the hydrogen atoms (each bonded to a lone pair on oxygen in another molecule).
• 2 bonds from the lone pairs on oxygen (each bonded to a hydrogen in another molecule).
Each ethanol molecule can form 2 hydrogen bonds:
• 1 bond from the hydrogen atom attached to the –OH group
Oxygen lone pairs (2 bonds):
• 2 bonds from the oxygen atom with 2 lone pairs.
The ethyl (C₂H₅) group doesn’t participate in hydrogen bonding, so weaker forces overall
How would you expect acetone to evaporate? (3)
IMF type: Mainly dipole-dipole and London dispersion forces.
No hydrogen bonding.
Weakest IMF of the three.