ras and other oncogenes
1/38
Earn XP
Description and Tags
lectures 3 and 4
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
39 Terms
Who was Robert Weinberg and what did he discover?
Discovered that cancer-causing genes could be transferred directly from cancer cells to normal cells
could bypass RSV into cancer genes
cancer cells make normal cells proliferate
Describe Weinberg’s experiment
solution with DNA and calcium chloride is added to HEPES buffered saline solution →
DNA precipitated out of the solution →
solution with precipitate (DNA binds to calcium and forms tiny white particles) is added to cells →
particles are ingested by cells, and would therefore ingest DNA bound to it.
Who was Shih? What did he do?
Graduate student in Weinberg’s lab
Transferred mouse cancer cell DNA to normal cells and they grew in foci (clumps) → cancer!
Moved to confirm in human cells
What was the first non-viral human oncogene?
Ras
native from cancer cells
functions as a GTPase, similar to ATP energy molecule
Describe the race to isolate and identify the first the first non-viral human oncogene.
3 groups isolated the same gene: Weinberg (MIT), Barbacid (NCI), and Wigler (Cold Spring Harbor NY) and published findings in Nature.
all isolated same gene: Ras!!
had also been discovered in a virus before (“Ras” = rat sarcoma).
In cell cultures, how do normal cells proliferate compared to cancer cells?
normal cells = monolayer with contact inhibition
FLAT, extended morphology
cancer cells = no contact inhibition, continue to grow in clumps/foci
can grow in low serum
ROUND morphology
anchorage independence: can grow without attaching to a surface
How does normal Ras compared to mutated Ras?
Ras in normal cells: TIGHTLY regulated
Ras in mutated cells: HYPERACTIVE and always on
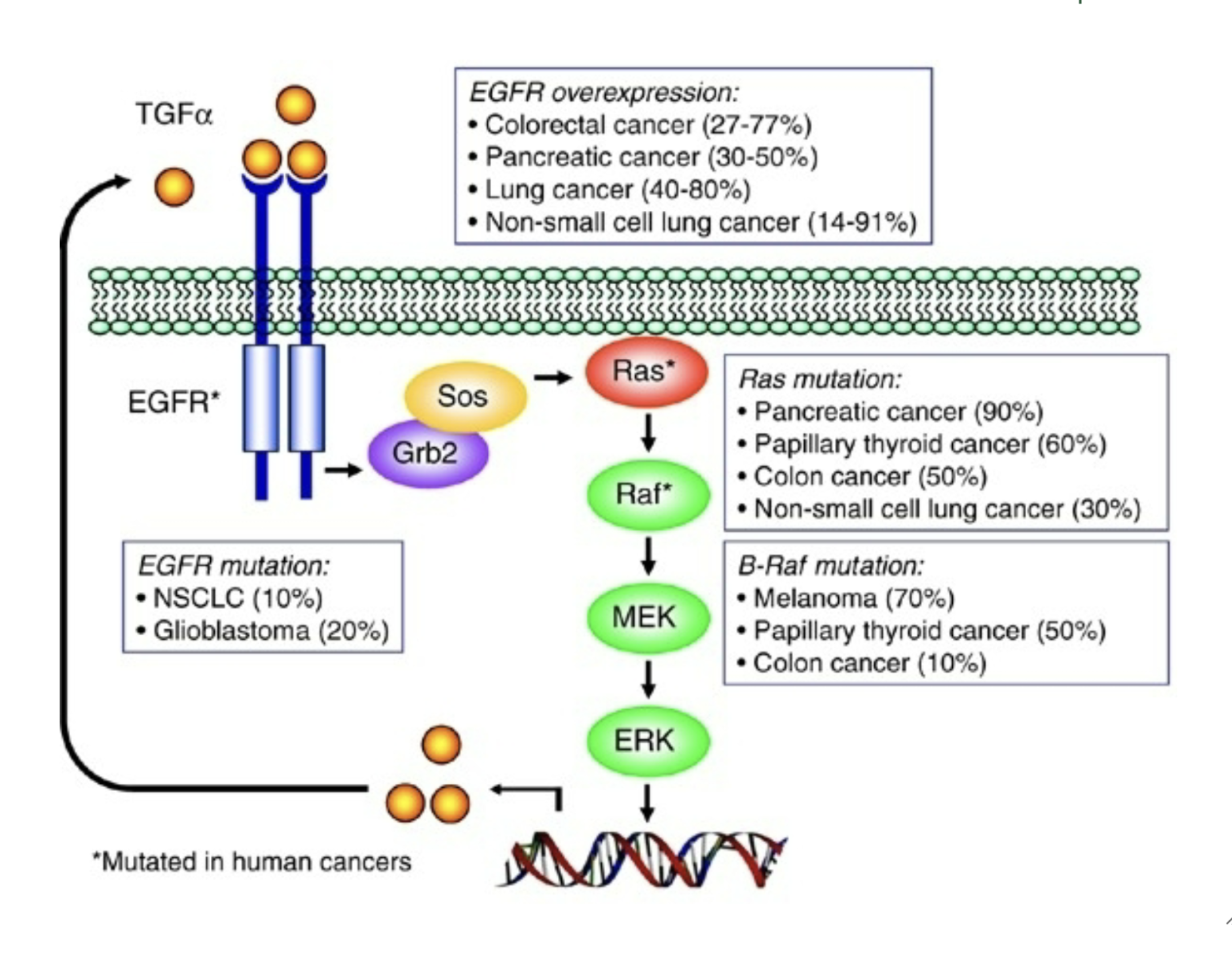
Explain Ras cascade
TGFa binds to EGFR on cell surface →
EGFR activates and then recruits Grb2 →
Grb2 recruits Sos →
Sos recruits Ras →
Ras recruits Raf →
Raf recruits MEK →
MEK recruits ERK →
ERK enters nucleus and promotes cell division
What is Myc short for? What does it cause in chickens?
Myelocytomatosis causes leukemia and sarcoma in chickens
discovered as v-myc oncogene (virus causes myelocytomatosis)
it’s a transcription factor and it binds to regions of the DNA to promote transcription (makes RNA which then gets translated to protein)
it’s one of the most highly amplified oncogenes in several cancers (colon, lung, stomach, cervix)
Human Myc is consistently altered by chromosomal translocation in which 2 cancers?
Burkitt Lymphoma (B-cell lymphoma)
Multiple myeloma (plasma cell cancer)
Define transgenic mice
introducing exogenous genes into mouse embryo
What is the OncoMouse (c-Myc)?
1988, Leder and Stewart
first animal to be patented
c-Myc only developed small breast tumors and not in every mouse
over expressed Myc only in breast mammary cells, to specifically study over-expression of Myc and if it resulted in breast cancer
What was significant about Leder’s second OncoMouse?
activated 2 oncogenes: ras and c-myc
multiple tumors sprouted within months
cancer had artificially been created in an animal through altering endogenous innate genes!
Who is Lakshmi Charan Padhy? What did he do?
postdoc in Weinberg’s lab 1982
isolated oncogene from rat tumor - neuroblastoma
called the oncogene Neu
What is the human homolog of the Neu gene?
Human EGF Receptor (HER) AKA Her2
member of EGFR family of receptors (extracellular domain on the membrane)
Ras, Myc, Src, Bcr-Abl are al INTRAcellular unlike Her2/Neu
Which is more aggressive, Her2 positive or Her2 negative? Who discovered this?
Slamon discovered that Her2 was increased in ~20% breast cancer samples
Her2 positive is more aggressive
What’s the name of the antibody drug to target outside domain of Her2?
Genentech developed Trastuzumab - Herceptin
Herceptin: Her2 intercept and inhibitor
first cancer inhibitor drug
What does PI3K stand for?
Phosphoinositide-3 Kinase
What’s an example of a Class I Catalytic subunit for PI3K?
110a, also known as PIK3CA
HIGHLY MUTATED IN CANCERS
What’s an example of a Class I Regulatory subunit for PI3K?
85
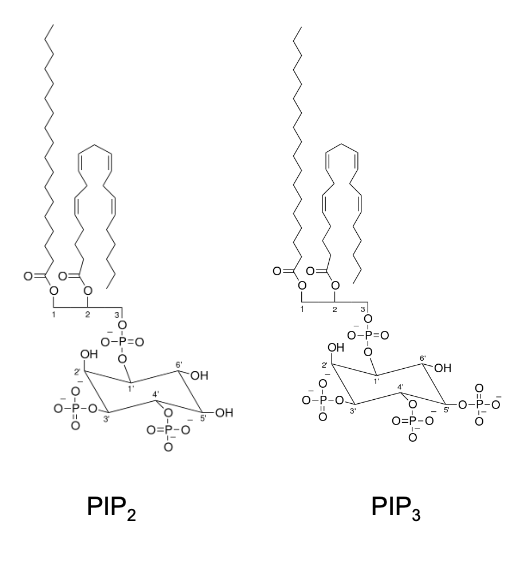
What does PI3K convert? What does this do?
PI3K converts PIP2 → PIP3 by phosphorylation
PIP3 activates PDK 1
PDK 1 activates AKT (phosphorylation) and mTOR activity
This ultimately promotes cell proliferation
What does PTEN do?
Reverses the path of PI3K by converting PIP3 back to PIP2 by removing the phosphate group (dephosphorylation).
What is PIP?
phospholipid that resides in the plasma membrane
What’s the difference between PIP2 and PIP3?
PIP2 = Phosphatidylinositol (4,5)-bisphosphate
PIP3 = Phosphatidylinositol (3,4,5)-trisphosphate
substrate for other kinases that promote proliferation
Which mutation of PIK3CA affects mobility of the activation loop?
H1047R
Generally, what do PIK3CA mutations do to the kinase?
Keep the kinase on or unable to turn the kinase off.
leads to the increase of PIP3 conversion and the downstream signals continue
Which cancers are caused by PIK3CA mutations?
breast and colon cancer
What are the 3 family members of Ras oncogene?
KRas
Kirsten murine sarcoma virus
HRas
Harvey murine sarcoma virus
NRas
Neuroblastoma Ras
What gets mutated in a mutated KRas gene?
P-loop
causes inability to conformationally change back to inactive state
What does KRAS need to be activated?
GTP
guanosine 5 triphosphate
GTP similar to ATP
What is the most common oncogene mutated in cancer?
KRAS
present in ~25-30% of all tumors
What cancer is KRAS most responsible for?
PANCREATIC (90%)
but also lung (32% and colon (40%)
What are the 2 most common mutations in KRAS?
G12C (lung) and G12D (pancreatic)
c = cigarrette = lung
d = diabetes = pancreas
Is GTP always necessary for KRAS mutations?
No, most mutations lock KRAS in active state and GTP is unnecessary.
Structural change → bind other proteins → downstream signaling without needing to be
activated itself.
What does EGFR stand for
epidermal growth factor receptor
commonly mutated or amplified in cancers
EGFR is most often in which 2 cancers? What do these mutations depend on?
Lung: kinase domain
Brain (glioblastoma): extracellular domain/ligand binding
In PIK3CA, the E542K somatic mutation has what percent of p110 mutations?
12%
this is a helical mutation
In PIK3CA, the E545K somatic mutation has what percent of p110 mutations?
24.9%
this is a helical mutation
In PIK3CA, the H1047R somatic mutation has what percent of p110 mutations?
40%
this is a kinase mutation