Unit 3 Energy Transformations
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
64 Terms
metabolism
the totality of an organism’s chemical reactions
metabolic rate
the total energy an organism uses over time
smaller organisms have a ? metabolic rate
higher
smaller organisms have greater SA:V ratio so they lose more energy
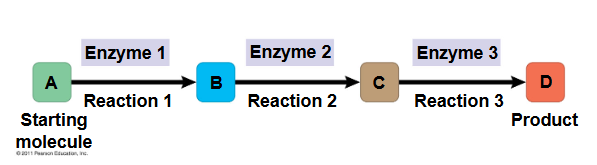
metabolic pathway
begins with a specific molecule and ends with a product
each step is ctalyzed by a specific enzyme
catabolic pathways
release energy by breaking down complex molecules into simpler compounds
ex: digestive enzymes break down food → release energy
anabolic pathways
consume energy to build complex molecules from simpler ones
ex: amino acids link to form muscle energy
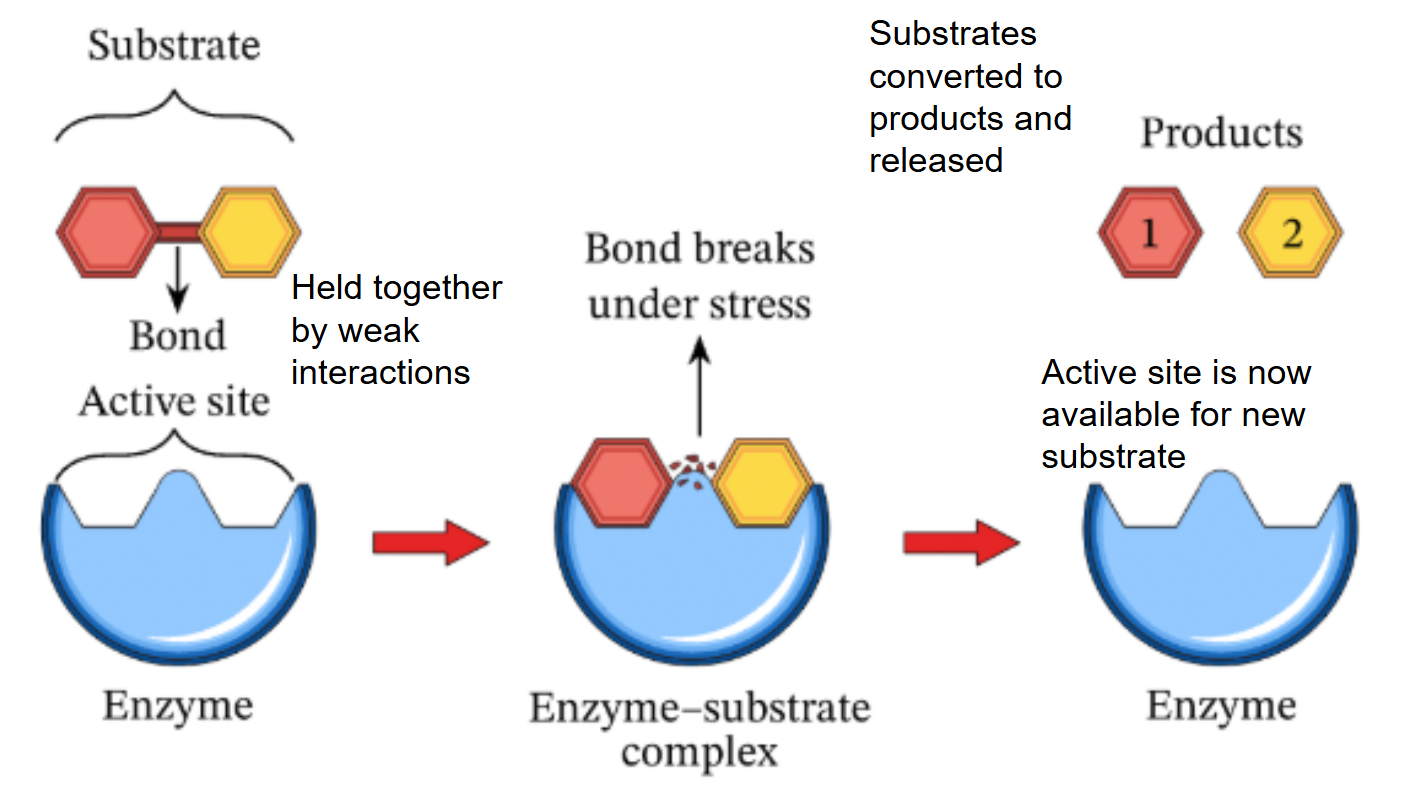
enzyme-substrate complex
every chemical reaction between molecules involves bond ? and bond ?
breaking, forming
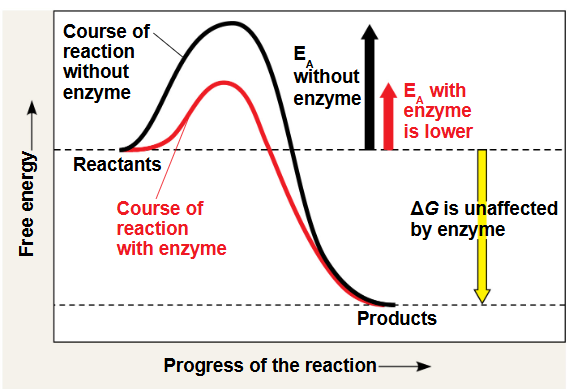
activation energy
the initial energy needed to start a chemical reaction is called the free energy of activation
how activation energy plays in a reaction
enzymes catalyze reactions by lowering the activation energy barrier
free energy (ΔG)
represents the energy available in a system to do work.
what does positive or negative free energy mean?
ΔG < 0 = exergonic reaction (spontaneous, no need for additional energy input)
ΔG > 0 = endergonic reaction (nonspontaneous, reqiires energy input)
ΔG = 0 equilibirum
products of a reaction are at a ? free energy state than reactants
lower
energy
capacity to cause change
Kinetic energy is energy associated with motion
Heat (thermal energy) is kinetic energy associated with random movement of atoms or molecules
Potential energy is energy that matter possesses because of its location or structure
Chemical energy is potential energy available for release in a chemical reaction
thermodynamics
study of energy transformations
isolated system
no interactions with environment
open system
energy and matter can be transferred between the system and its surroundings
ex: organisms
first law of thermodynamics
energy of the universe is constant
energy can be transferred and transformed, cannot be created or destroyed+
second law of thermodynamics
every energy transfer or transformation increases the entropy of the universe. this si because during every energy transfer or transformation, some energy is unusable, often lost as heat
entropy
a measure of disorder or randomness within a system
spontaneous processes
occur without energy and can happen quickly or slowly
means energetically favorable = -ΔG = increased entropy = more stability of system
energy flows into an ecoystem in the form of ? and exits in the form of ?
light, heat
exergonic reaction
net relase of free energy
spontaneous
endergonic reaction
absorbs free energy from its surroundings and is nonspontaenous
changes in free energy in a cell
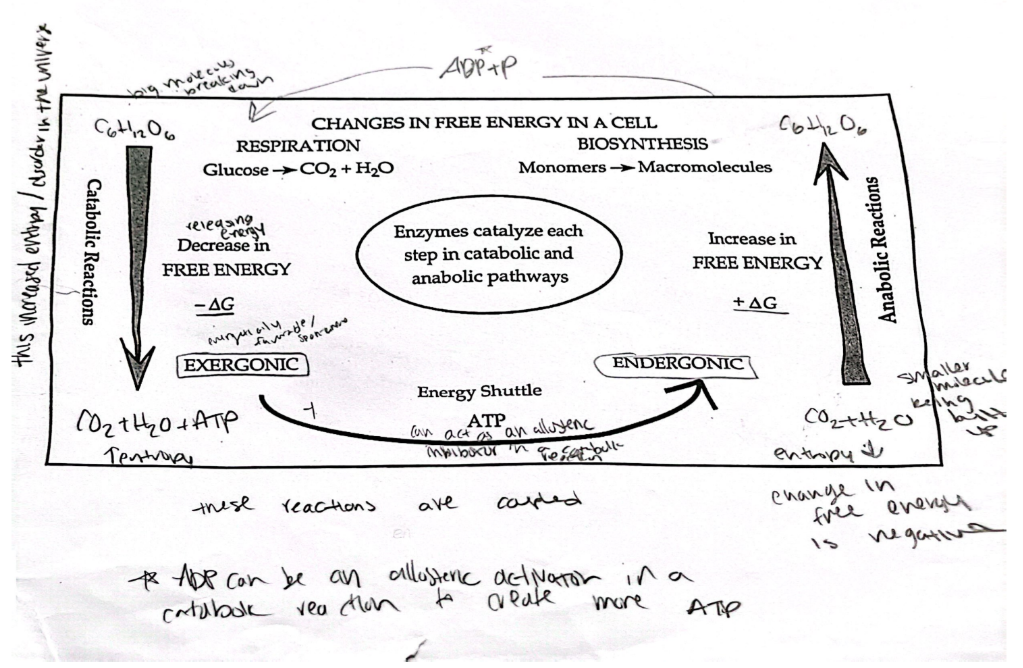
in catabolic reactions, the products have ? energy than reactants
in anabolic reactions, the products have ? energy
lower, higher
energy coupling
the use of an exergonic process to drive an endergonic one
most energy coupling in cells is mediated by ATP
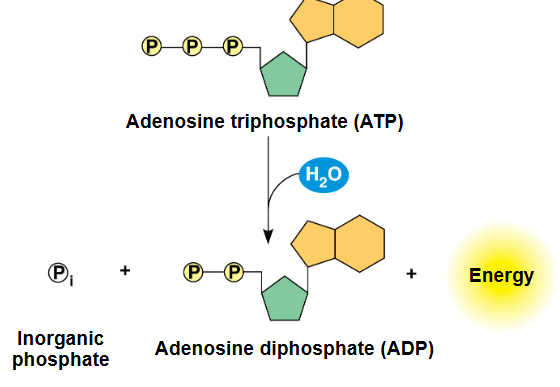
atp hydrolysis
catabolic/exergonic reaction where a molecule of ATP is broken down into ADP + P + energy
addition of water molecule breaks a high-energy phosphoanhydride bond between the second and third phosphate groups whic is catalyzed by the enzyme ATP hydrolase
released energy can be used for vaerious cellular processes
atp drives energonic reactions by ?
phospholryation which then changes shape and becoems more reactive
atp regeneration
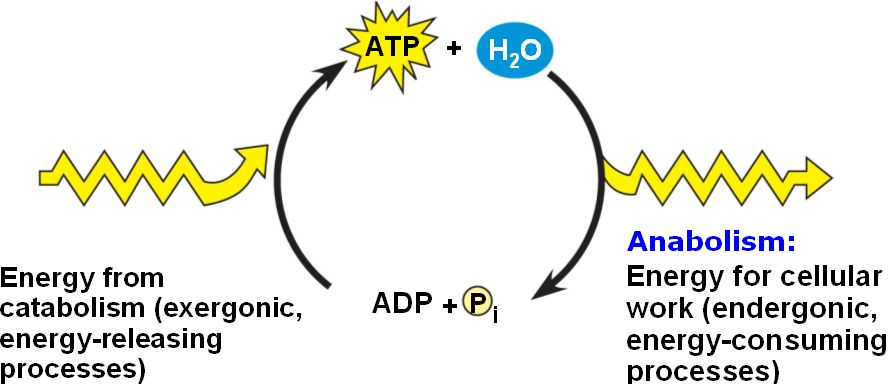
enzyme
catalytic protein
induced fit
substrate brings chemical groups of the active site into positions that enhance their ability to ctayalyze the raction
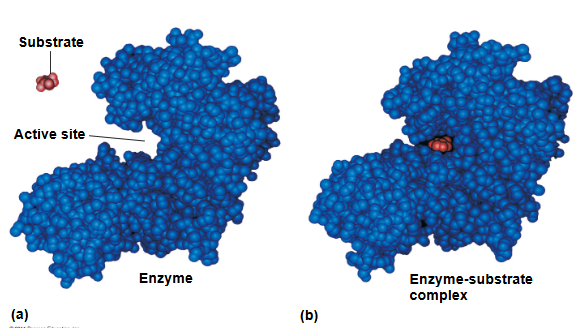
active site can ? an EA barrier
lower
factors that can affect an enzyme’s activity
General environmental factors, such as temperature and pH
Chemicals that specifically influence the enzyme
optimal conditions favor the most active shape for the enzyme molecule
optimal temp for human enzyme
37 degrees celsius
opyimal ph for stomach enzyme pepsin and optimal ph for trypsin (intensinal enzyme)
2, 8
cofactors
nonprotein enzyme helpers; help to catalyze reactions
competitive inhibitors
bind to the active site of an enzyme, competing with the substrate
to compete with his, more substrate is needed to outcompete with inhibitor
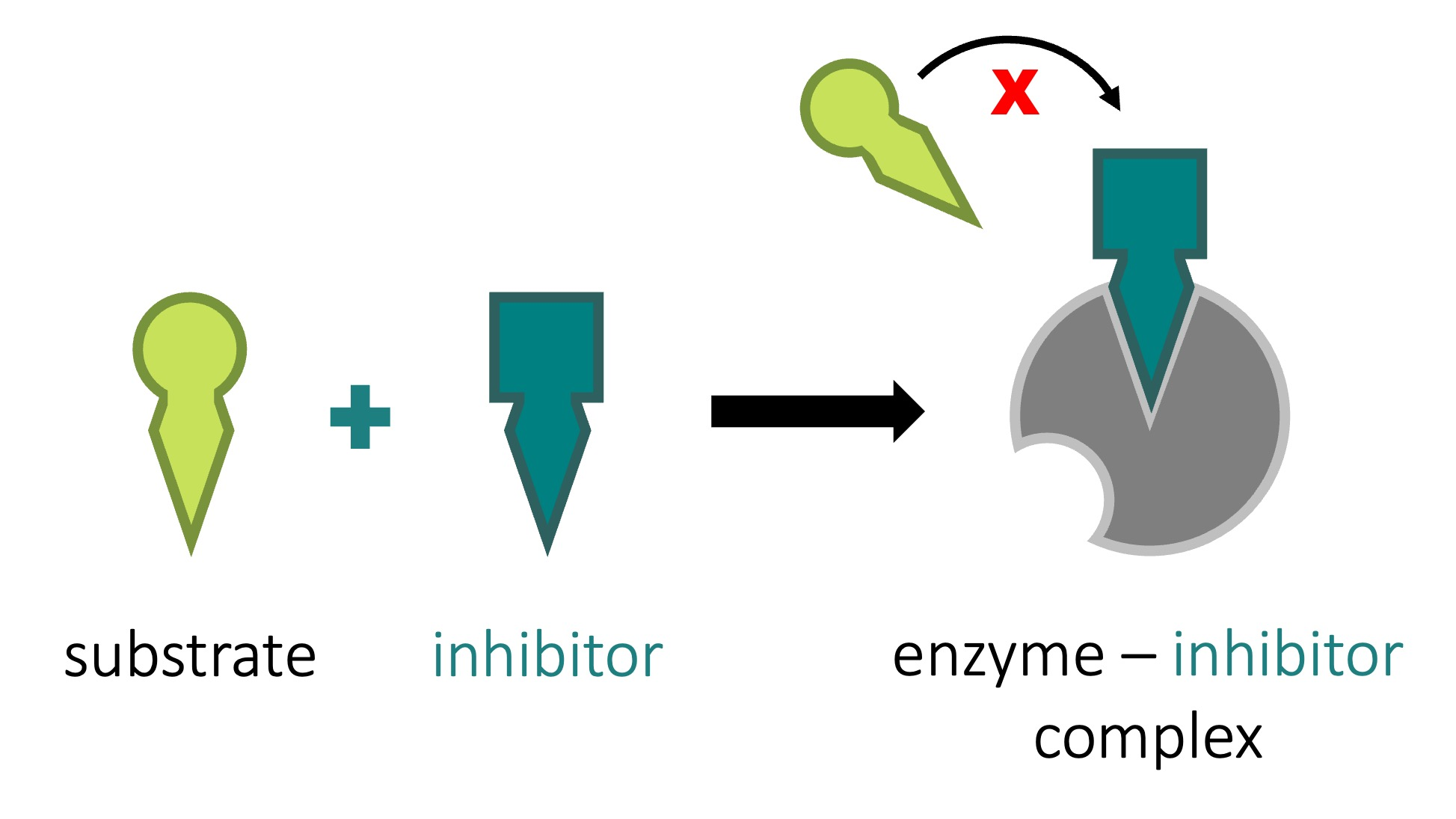
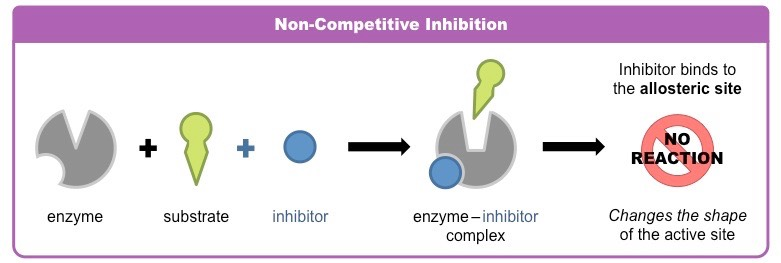
noncompetitive inhibitors
bind to another part of an enzyme, causing the enzyme to change hsape and making the active site less effective
ex: toxins, poisons, pesticifes, and antibiotics
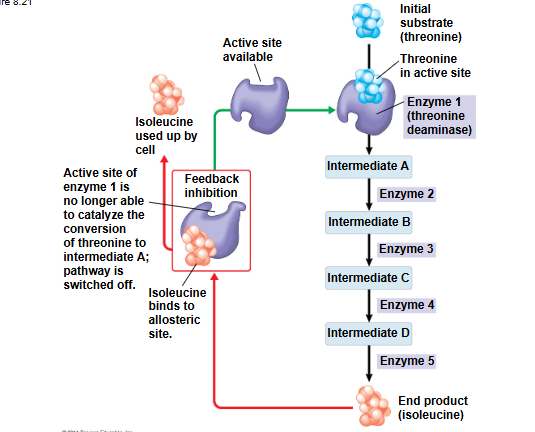
allosteric regulation
may either inhibit or stimulate an enzyme’s activity
occurs when a regulatory molecule binds to a protein at one site and affects the protein’s function at another site
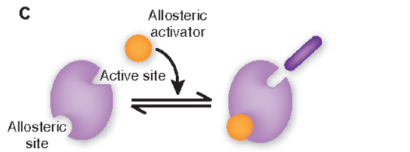
allosteric regulators as activators
activators bind to allosteric site and change the shape of the enzyem so the usbstrate now fits in enzyme
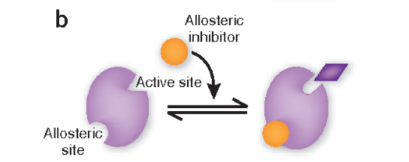
allosteric regulators as inhibitors
inhibitors bind to allosteric site and change the shape of the enzyme so substrate can’t bind to active site
feedback inhibition
end product of a metabolic pathway shits down the pathway
prevents a cell fromw asting chemical resources by synthesizing more product than is needed
cooperativity
form of allosteric regulation that can amplfiy enzyme activity
One substrate molecule primes an enzyme to act on additional substrate molecules more readily
Cooperativity is allosteric because binding by a substrate to one active site affects catalysis in a different active site
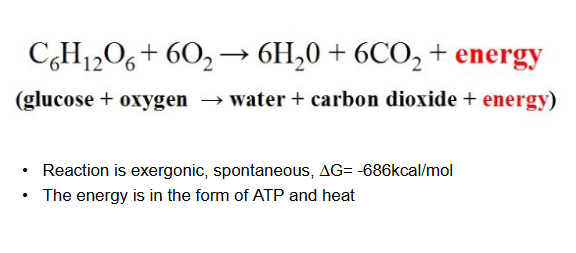
aerobic respiration
cellular respiration
oxygen required
more energy released through the oxidation of glucose
anaerobic respiration
fermentation
no oxygen required
less energy released through partial splitting of glucose
aerobic pathway
glycolysis
first step in cellular respiration
occurs in the cytosol
turns glucose into 2 3-C sister pyruvates/pyruvic acids
takes 2 atp to start , forms 4 atp
NET total: 2 pyruvates + 2H20, 2ATP, 2 NADH + 2H
redox reaction
allows energy to be released from glucose in a series of steps that will capture the maximum amount of energy that is released from it
electrons lose energy as they’re transferred from a less to a more electronegative atom
coenzyme NAD+ will serve as an intermediate electron acceptor throughout the process and FAD serves as an electron carrier as FADH2
NADH will eventually give up the electrons to the ETC and they will gradually be transferred to a lower energy level (through oxidation/reduction) and their energy will be harnessed
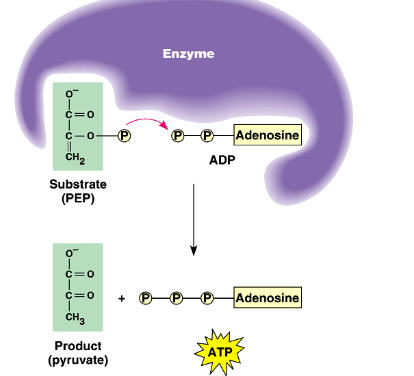
substrate level phosphorylation
a biochemical reaction where a phosphate group is directly transferred from a high-energy phosphorylated intermediate to ADP (or GDP) to produce ATP (or GTP)
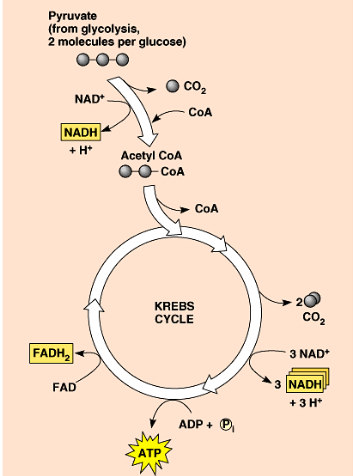
what happens after glycolysis but before enetering the citric acid cycle?
2 pyruvates are transformed to 2 actetyl coA
through NAD+ → NADH and removal of Co2 and addition of coenzyme A
citric acid cycle (krebs cycle)
occurs in the mitochondrial matrix
acetyl coa enters the cycle and is added to oxaloacetate to produce citrate
through redox reactions, 2 ATP is produced