Chemistry 202 Class 5- Le Chatelier's Principle & the Connection Between Free Energy and Equilibrium
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
Increasing concentration of reactants…
Reaction will shift to the right (forward) in order to “use up” the additional reactant, producing more products
Increasing concentration of products…
Reaction will shift to the left (reverse) to use up the added product
Decreasing concentration of reactant…
Reaction will shift left (reverse) to produce more reactants
Decrease concentration of products…
Reaction will shift right (forward) to produce more products
Endothermic and Changing T
K increases as T rises. Why? Think of heat as a reactant- by adding heat, they system will move forward to make more products
Exothermic and Changing T
K increases as T decreases. Why? Think of heat as a product- by removing heat, the reaction will more forward to produce more products
Catalyst and Equilibrium
Catalysts do not impact the position of equilibrium, they can only speed up how fast a reaction reaches equilibrium
Decrease in volume…
The equilibrium will shift to favor the direction that produces fewer moles of gas
Increase in volume…
The equilibrium will shift to favor the direction that produces more moles of gas
Van’t Hoff formula
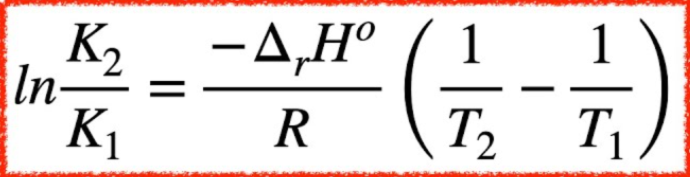
Van’t Hoff, K, and T
Van’t Hoff allows us to solve for ΔH, which tells us if the reaction is exo or endothermic. With this, we can determine how K will change with changes in T
Standard State
P= 1 atm
T= 25 C (298.15 K)
All concentrations at 1M
Q at standard state
Q=1 AT STANDARD STATE