m8 tot
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
H.V. Wilson Sponge Experiment
First demonstrated the ability of cells to recognize and adhere to one another
Used the cells of 2 sponge species
Their indiv cells were seperated using a fine mesh
The cells were then mixed together
Overtime, the cells from the same species were able to recognize and associate back together
Cells from diff species didn’t associate
Johannes Holtfreter: Frog Embryo Experiment
Showed cell recognition and adhesion using frog embryos
Took cells from 2 different developmental germ layers and seperated indiv cells
Similar tissue recognized eachother and associated
The associations mimicked original embryo organization
What are the three developmental germ layers of an early embryo
Endoderm
Ectoderm
Mesoderm
During embryogenesis, how do cells recognize and stay together?
Requires transmembrane proteins called CAMs (cell adhesion molecules)
After aggregation, they form specialized junctions stabilizing the cell interactions
Facilitated communication between adjacent cells
How are epithelial cells organized, and what do they form in the body?
Epithelial cells connect along their lateral surfaces to form epithelial sheets.
These sheets line body cavities and cover surfaces like the digestive tract and skin.
Each epithelial cell has distinct surfaces:
Apical surface: faces the lumen or outside (e.g., with microvilli in the intestine).
Basal surface: faces inward and is attached to the basal lamina / basement membrane.
What connects epithelial cells to the underlying extracellular matrix?
The basal surface anchors to the basal lamina (basement membrane).
Hemidesmosomes are adhesion complexes that connect the cell’s basal side to the extracellular matrix (ECM), providing structural support.
Which types of adhesion complexes connect the lateral surfaces of epithelial cells?
Tight junctions
Adherens junctions
Desmosomes
Gap junctions
Tight Junctions
zonula occludens
Connect adjacent cells below the apical surface
It completely seals the space between the cells
Prevents fluid from moving across the layer
Restricts diffusion of small molecules in gastrointestinal track to prevent enzyme leakage
Done by linear arrays of occludin and claudin
‘Pinches’ cells together
Gap Junction Function
Link the cytosol of one cell to the other
Allows for integration of metabolic activities of all cells in a tissue by allowing ion/small molecule exchange
Ex. cAMP and Ca++
Diameter of Gap Junction Channels
1.5-2 nm
Allows for free diffusion of molecules up to 1 kDa in size
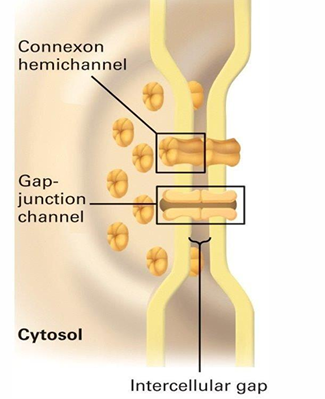
Gap Junction Structure
6 connexin proteins make a hexagonal connexon hemichannel
One hemichannel will sit in the cell membrane of each connected cell
Two lined-up hemichannels form a gap junction
These hemichannels are found in groups to form gap junction rich regions
Gap Junction Applications
Allows for diffusion convenient for interconnected cells
rapid coordination of cardiac muscle contraction
rapid uterine muscle contraction
Stimulation of one cell leads to a response shared by many cells through diffusion of secondary messangers
Gap Junctions in Plant Cells: Plasmodesmata
Important to the structure and function of phloem
Phloem is a system of tubes formed by cells connecting linearly
It carries nutrients to the rest of the plant
Sieve-tube elements are connected by plasmodesmata that form the seive tube plate
They’re metabolically inactive
Companion cells provide ATP and substances to these cells
They’re also ocnnected by plasmodesmata
Plasmodesmata also helps with communication through informational molecules
Gene transcripts, small RNA, etc.
Pathogens also exploit this though
Anchoring Junctions
Includes:
Adherens junctions
desmosomes: Link 2 cells together
hemidesmosomes: Attach cells to extracellular matrix
Distinguished by their association with actin filaments
Through the connections, adherens junctions indirectly connect the actin cytoskeleton between neighbouring cells
Four Families of Cell Adhesion Molecules that Make up Adherens Junctions
Cadherins
Ig-superfamily
Integrins
Selectins
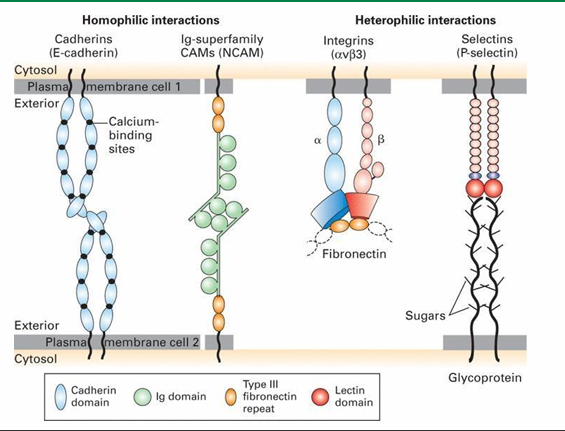
Which cell adhesion families form homophilic interactions?
This means association of similar cells
Cadherins
Ig-superfamily CAMs
Which cell adhesion families form heterophilic interactions?
This binds non-similar cells
Integrins
Selectins
Cadherins Function
Cell adhesion molecules of adherens junctions
They’re calcium dependent CAMs mediating homophilic interactions
They mediate epithelial cell adhesion near the apical surface
What are the three major classes of cadherins
E-cadherin (epithelial)
N-cadherin (neural)
P cadherin (placental)
Cadherins Mechanism
Adhesion involves
transmembrane cadherins
cytosolic cofactors
catenins (anchors cadherin to actin)
Cells do not aggregate into sheets under standard cell conditions
E-cadherin must be expressed
It’s calcium-dependent
Without calcium, E-cadherin cannot function, and cells remain separate.
Extravasation
The movement of WBC from blood stream to surrounding tissue
5-step process initiated by a signal created by infection
Why are transient (temporary) cell adhesions important, and when do they occur?
Not all cell adhesions are permanent — some are temporary to allow movement.
Transient adhesions are essential for:
Cell migration across extracellular surfaces.
Cell movement during embryogenesis.
These connections form and break repeatedly, enabling cells to travel where needed.
How do leukocytes use transient adhesion during an immune response?
Leukocytes must exit blood vessels to reach sites of infection or injury.
This extravasation relies on a sequence of temporary adhesive interactions with endothelial cells
Normally, adhesion between endothelial cells prevents blood leakage
During an immune response, leukocytes temporarily attach and cross the vessel wall to enter tissues.
What are the 3 families of WBCs / Leukocytes
Granulocytes: Neutrophils
Monocytes: Macrophages
Lymphocytes: T and B cells
Granulocytes
Target pathogens
Include neutrophils, eosinophils, and basophils
Neutrophils
Most common granulocyte
Primarily targets bacteria infections
One of the first cells to respond to trauma
Capable of extravasation
Monocytes
They differentiate into microphages
They engulf invading bacteria or dead cells through phagocytosis
Capable of extravasation
Lymphocytes
Include NK (natural killer) cells
Lyse virally infected cells and tumour cells
Include T and B cells
Produce antibodies as immune response
Can undergo extravasation
What are the five steps of extravasation
capture
rolling
slow-rolling
firm adhesion
transmigration
Extravasation: Step 1
Capture (Using Neutrophil Ex)
This is the transient association between the neutrophil and the apical surface of endothelial cell
They’re still being pushed by bloodflow but slower
The cells roll along the surface of endothelial cells
Extravasation: Step 2 / 3
Rolling / Slow Rolling
Since the transient associations are slowing the neutrophil, it rolls along the surface
The rate slows down as # of associations increase
This leads to firm adhesion
Extravasation: Step 4
Firm adhesion
Occurs with stronger attachment of neutrophil with endothelial cells
This is accompanied by changes allowing the WBC to break connections b/w endothelial cells
This allows migration along the cell surface to outside the blood vessel
Extravasation: Step 5
Transmigration
The seperation of endothelial cells allow the neutrophil to migrate out of the blood vessel
Causes swelling as transmigration occurs
Extravasation Capture Mechanism
Cytokines (e.g., TNF-α) are released at the infection site
They signal endothelial cells of blood vessels.
This signal (received at the basal surface) triggers endothelial cells to move P-selectins from secretory vesicles to their apical surface.
P-selectins on the endothelial surface then bind to selectin-specific glycoprotein ligands on neutrophils
This captures them from the bloodstream and initiates the immune response.
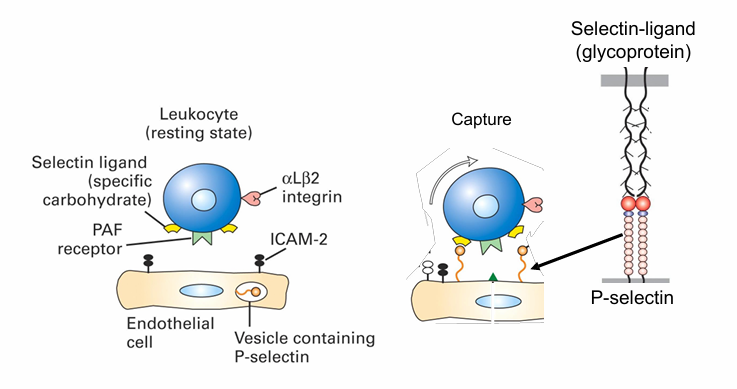
Extravasation Rolling Mechanism
Adhesion of neutrophil to endothelial cells slow movement
Eventually, they start rolling along the walls
This involves them being pushed over the surface while establishing and losing transient connections
Extravasation Slow-Rolling Mechanism
Density of selectins on endothelial cells inc closer to site of infection
Many endothelial cells are displayed P and E selectin here
The inc associations between selectins and the ligands on neutrophils flows their movement
They are no undergoing slow-rolling
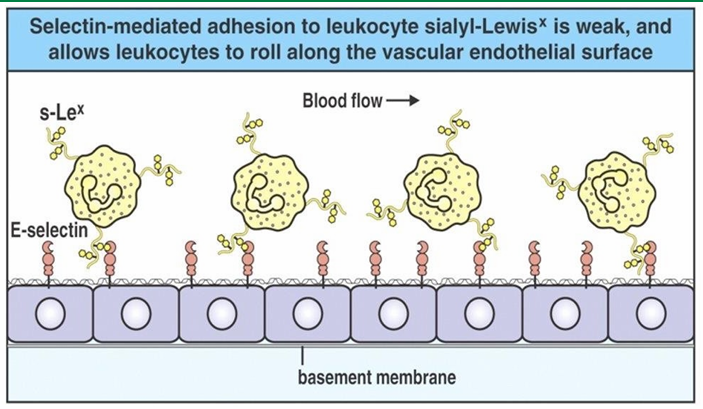
Extravasation Firm Adhesion Mechanism
Slow rolling lets new interactions form between neutrophils and endothelial cells.
PAF (platelet activating factor) on endothelial cells binds to the PAF receptor on neutrophils (a
Ex. receptors CXCR1 and CXCR2
This interaction occurs only during slow rolling and activates a signal transduction pathway inside the neutrophil.
The signal activates integrin adhesion molecules on the neutrophil, enabling them to bind ICAMs on endothelial cells.
This binding slows the neutrophil further, leading to firm adhesion (tight binding) to the vessel wall.
Integrin Protein Structure (Extravasation Firm Adhesion)
Inactive integrin (dimeric) has its propeller and β-A domains folded down, preventing ligand binding.
PAF signaling triggers a conformational change, activating the integrin so it can bind ICAMs on endothelial cells.
Integrin–ICAM binding is much stronger than selectin interactions, resulting in firm adhesion of the neutrophil.
Activation also initiates actin cytoskeleton reorganization, preparing the neutrophil for cell migration out of the blood vessel.
Extravasation Transmigration Mechanism
The neutrophil has stopped at the site of infection
It can migrate b/w the endothelial cells
The connections b/w them are broken by enzymes produced by transmigrating neutrophil
Progressive Activation of Extravasation
Selectins are activated first
Mediates capture, rolling, and slow-rolling
Signalling pathways activate integrins
Mediates firm adhesion
Allows transmigration