10 - Ethanol Metabolism
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
Describe the process of alcohol metabolism.
Alcohol is absorbed into the bloodstream through the mucosal lining of the gastrointestinal tract. 10% is evaporated, while the rested are metabolized by liver enzymes alcohol dehydrogenase and acetaldehyde dehydrogenase.
In the cytosol, alcohol dehydrogenase converts ethanol into acetaldehyde using one NAD+ to form NADH.
In the mitochondria, acetaldehyde dehydrogenase converts acetaldehyde to acetate using another molecule of NAD+ to form NADH.
Other pathways include CYP2E1 (not active unless large amounts are consumed) using NADPH to NADP+ in the microsome and by catalase in the peroxisome (H2O2 to H2O). both form acetaldehyde.
What are some of the effects of acetaldehyde?
Acetaldehyde is a toxic metabolite that causes common symptoms of drunkness.
It also causes damage of liver, brain. Pancreas, and GI tract.
It impairs memory, coordination, and causes sleepiness.
How is methanol toxic?
Methanol is toxic because it is metabolized in the same pathway as alcohol (to formaldehyde and then to formic acid)
It can cause ocular toxicity, including blindness, basal ganglia injury and other brain damage
How is ethylene glycol toxic?
Ethylene glycol is active in antifreeze and can be broken down into glyoxylic acid or oxalic acid which can lead to lactic acidosis and calcium oxalate (crystals can precipitate in proximal renal tube, leading to acute tubular necrosis and renal failure)
How can administering tylenol be toxic in conjunction with alcohol? What are the normal pathways of tylenol metabolism?
Acetominophen (tylenol) can be converted into O-sulfate and O-glucoronide to be excreted by urination.
When CYP2E1 is active, it can be converted into NAPQI (N-acetyl para-quinone imine), which can be excreted only when bound to glutathione to form mercapturate (urinated).
If there is not enough glutathione, NAPQI binds to hepatocytes and kills them.
If tylenol is taken with alcohol, more protein binding occurs and causes irreversible damage.
4 g is a toxic dose, and 15g necessitates liver transplant.
NAPQI buildup can be treated by increasing glutathione by administering its precursor, N-acetyl cysteine (NAC) (mucomyst).
How can mutations in aldehyde dehydrogenase protect against alcohol abuse?
Acetaldehyde dehydrogenase deficiency in people of Asian decent have a glutamate to lysine mutation in the gene encoding for aldehyde dehydrogenase 2 (ALDH2).
This decreases affinity for NAD+, leading to accumulation of acetaldehyde and alcohol flushing.
Leads to less likely to develop alcohol use disorder and higher rates of esophageal cancer.
How does 4-methylpyrazole protect against methanol poisoning?
4-methylpyrazole (fomepizol) can inhibit cytosolic alcohol dehydrogenase in protection against methanol poisoning.
Could also by treated by ethanol by competitive inhibition, leading to complete intoxication.
How does aversion therapy work?
Patients are given disulfiram, which inhibits acetaldehyde dehydrogenase, leading to an abundance of acetaldehyde. This could cause sickness and significant symptoms even for small amounts of alcohol. Some symptoms include hypotension, dyspnea, overactivty, palpitations, nausea, and vomiting.
What is the role of cytochromes?
Role of cytochromes is to make things that aren't water soluble, soluble by adding an OH (phase 1). Otherwise in phase 2 it is conjugated with a group
What are the effects of ethanol metabolism on gluconeogenesis?
Alcohol dehydrogenase and aldehyde dehydrogenase use one NAD+ to convert to NADH, increasing the NADH:NAD+ ratio, triggering activation of reactions that convert NADH to NAD+ (pyruvate to lactate, creating lactic sarcoidosis state preventing oxidation of lactate to pyruvate).
Conversion of oxaloacetate to malate via TCA cycle.
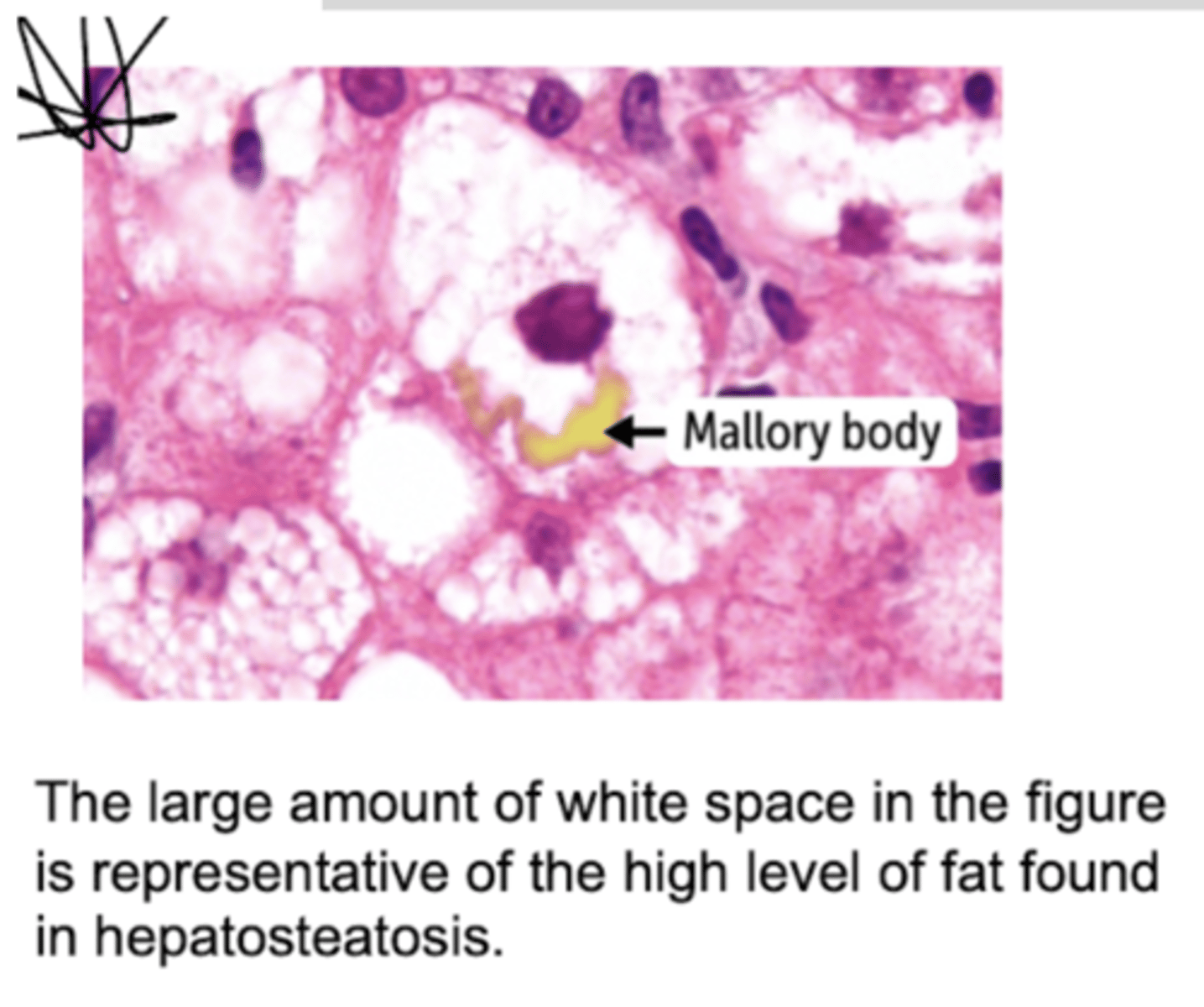
High ratio triggers inhibition of gluconeogenic dehydrogenase enzymes (leading to fasting hypoglycemia), leading to the conversion of DHAP to G3P, and G3P combines with FA to form TAG and leading to fatty liver. Most importantly, gluconeogenic consequence is the shunting of resources away from gluconeogenesis, which prevents the liver from making glucose during fasting, pursuing ketogenesis and resulting in anion gap metabolic acidosis.
What is anion gap, how is it measured, and how does it assist in different diagnosis?
The quantity difference between cations (+) and anions (-) in serum, plasma, or urine.
Magnitude of this difference in the serum is calculated to identify metabolic acidosis.
If gap is greater than normal, high anion gap metabolic acidosis diagnosed.
Calculated by subtracting the serum concentrations of chlorine and bicarbonate anions from the concentrations of sodium and potassium cations.
Uses of this: helps in differential diagnosis of number of disease states.
Total cations should equal anions so theres neutral charge
Routine tests do not account for all types of ions - is representative of how many ions are not accounted for by the lab measurements used in the calculation (unmeasured are mostly anions, hence named anion gap).
