10. molecular characteristics of good drugs (copy)
1/24
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
25 Terms
what is serendipity?
the effect by which one accidentally discovers something fortunate, especially while looking for something else entirely
examples of serendipity?
mustard gas
disulfiram drug
clonidine
sildenafil
orally- active drugs, what are the problems to overcome?
has to pass through the lumen of the gut
through the gut wall and beyond into the bloodstream (aqueous)
for orally active drugs to pass through the lumen of the gut wall and beyond into the bloodstream, what important factors do we have to consider?
size
solubility
hydrogen bonding
size
drugs that are successful for oral delivery tend to be?
small
(they can travel cross membranes better)
solubility
for a compound to cross a membrane it must be?
soluble in water and also lipid
(not too soluble in either)
solubility
log P is the usual parameter used to?
articulate solubility
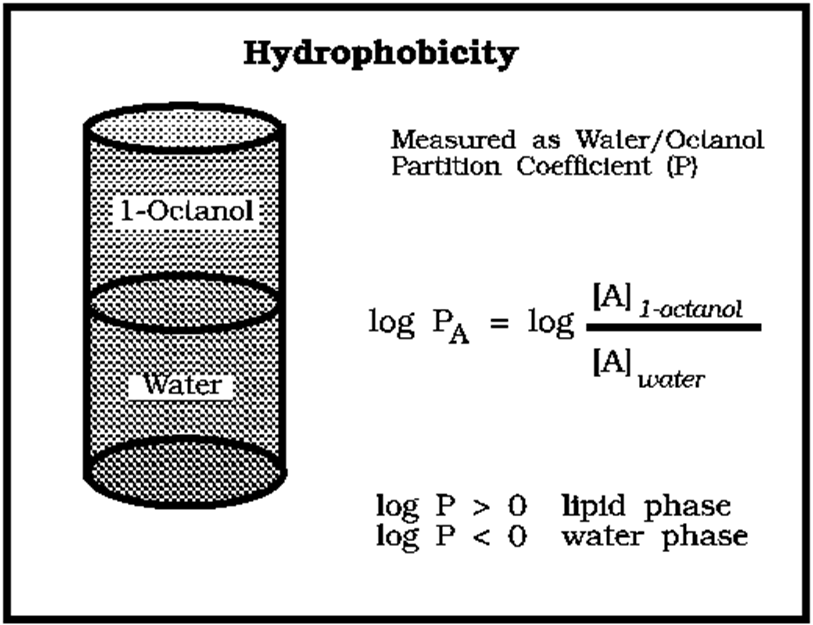
what does the P stand for in log P?
P is the partition coefficient
solubility
compounds with a log P value above 5 are very?
are very hydrophobic - sticky in membranes
HHYDROGEN BONDING
in lipinski rule of 5 are HBA and HBD required?
yes
HYDROGEN BONDING
do OH and NH act as HBA or HBD?
HBD
HYDROGEN BONDING
do O and N act as HBA or HBD?
HBA
lipinski’s rule of five
poor absorption (or permeation) is more likely when?
molecular weight is over 500
the log P is over 5
there are more 5 H bond donors (expressed as a sum of OH and NH)
there are more than 10 H bond acceptors (expressed as the sum of N and O)
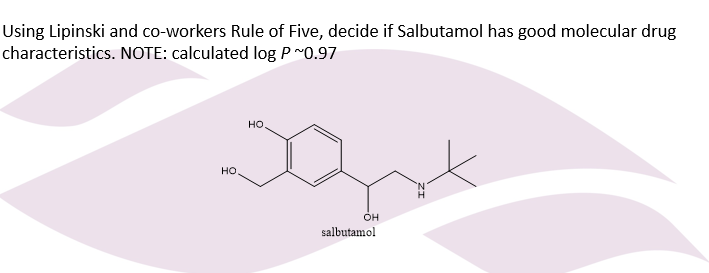
Lipinski Example?
Molecular Weight: The molecular formula for salbutamol is C₁₃H₂₁NO₃, giving it a molecular weight of approximately 239.3 g/mol. This is not over 500, so there is no violation.
log P: The calculated log P for salbutamol is ~0.97. This is not over 5, so there is no violation.
H Bond Donors: Salbutamol has three -OH groups and one -NH group, for a total of 4 hydrogen bond donors. This is not more than 5, so there is no violation.
H Bond Acceptors: Salbutamol has three oxygen atoms and one nitrogen atom, for a total of 4 hydrogen bond acceptors. This is not more than 10, so there is no violation.
Yes, based on Lipinski's Rule of Five, salbutamol has good molecular drug characteristics because it does not violate any of the rules.
Since salbutamol has 0 violations, it is predicted to have good absorption and permeation properties.
lipinski rule of 5 expections?
drugs for delivery via non-oral routes
drugs to treat disorders of the gut
antibiotics, antifungals, vitamins and cardiac glycosides
SARs
preparation of analogues in a logical systematic way…
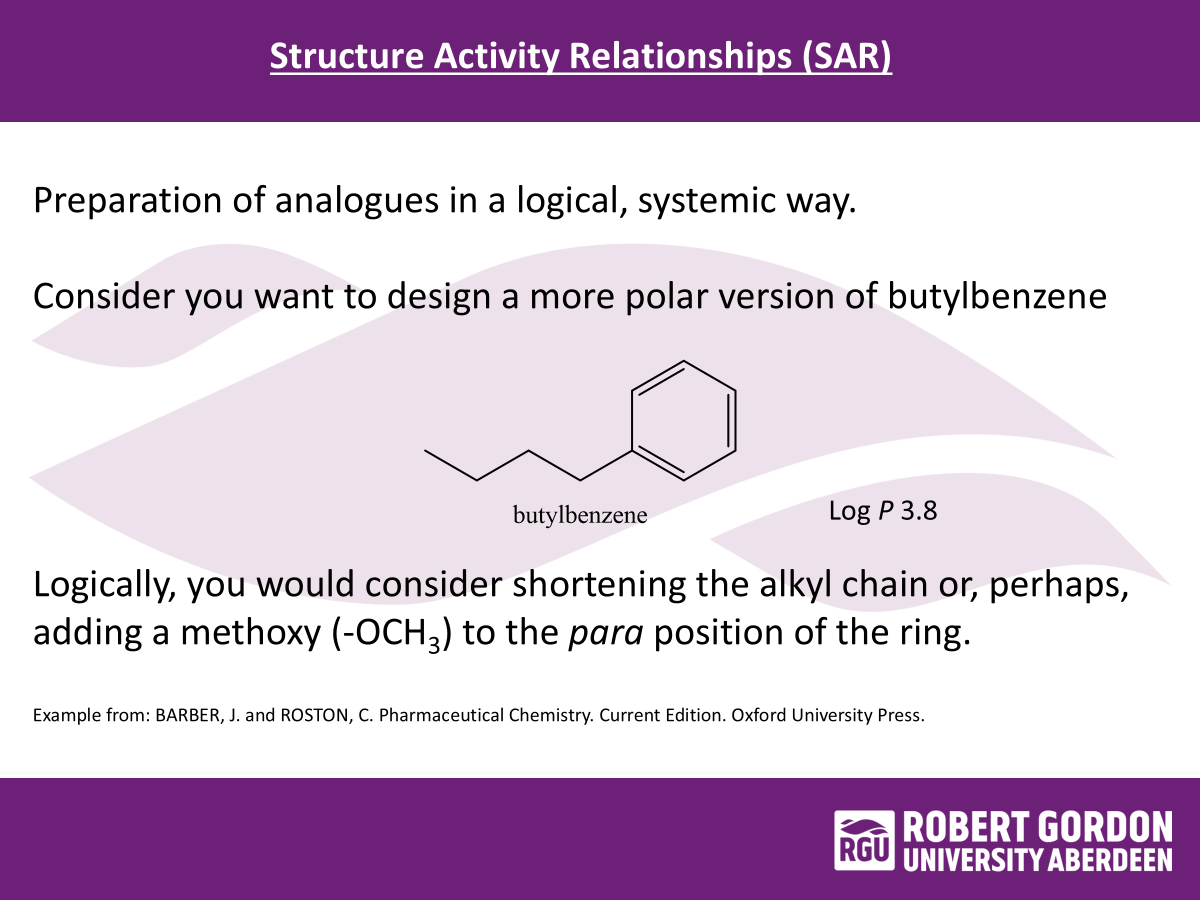
SAR example:
the IUPAC definition of a pharmacophore?
an ensemble of steric and electronic features
its necessary to ensure the optimal supramolecular interactions with a specific biological target
trigger (or block) its biological response
define quantitative structure-activity relationship (QSAR)?
is the process which a chemical structure is quantitatively correlated with a well defined process such as biological activity or chemical reactivity.
lead optimisation
how do medical chemists improve drug efficiency?
stability
bioavailability
e.g. developing mechanism- based pro-drugs
How are pro-drugs engineered?
In a way that they undergo chemical transformation in the bloodstream or specific tissues i.e liver.
Upon transformation, biologically active metabolites (actual drugs) are released
activity of sites for chemical derivatisation…
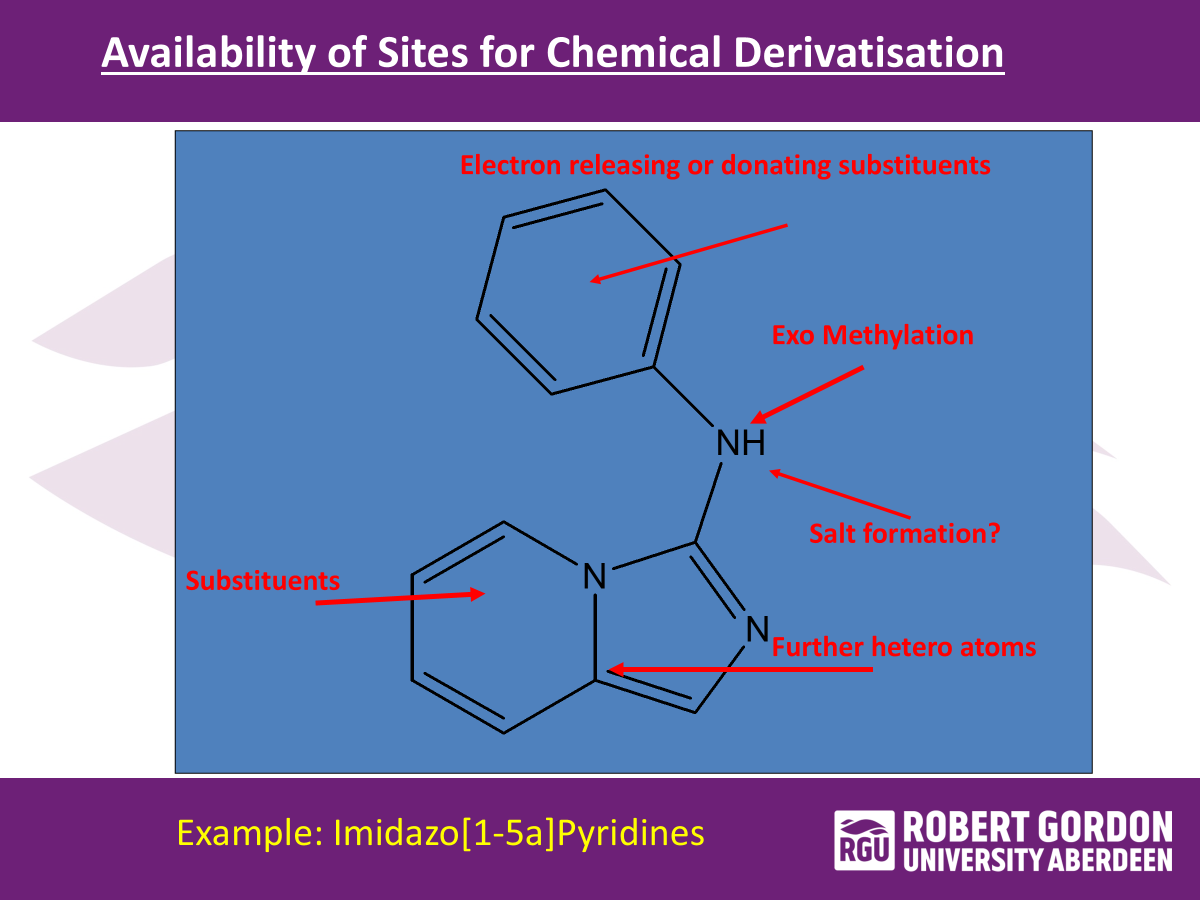
chemical manipulation
very few lead componds are ideal. most are likely to have low?
low
activity
poor selectivity
significant side effects
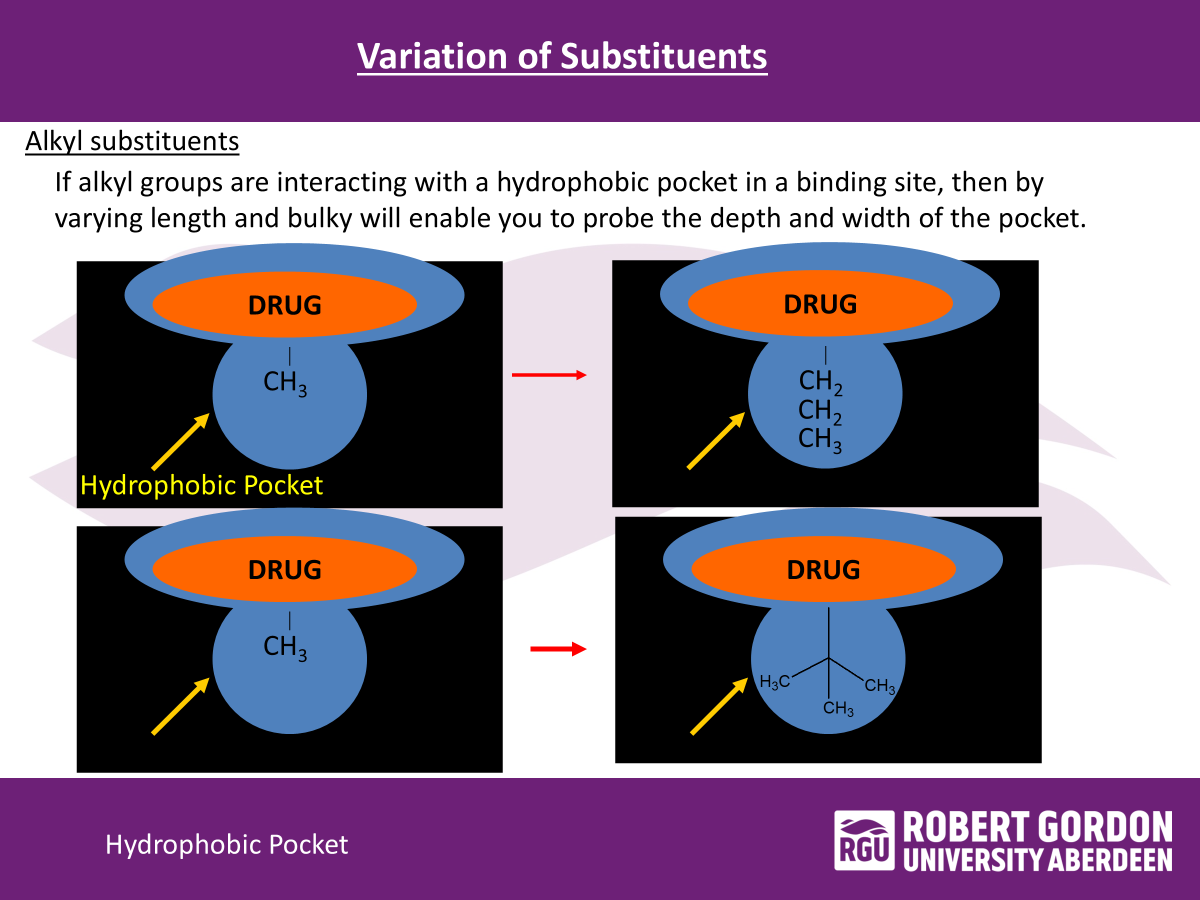
variation of substituents
if alkyl groups are interacting with a hydrophobic pocket in binding site, then by varying length and bulky will enable you to?
probe the depth and width of the pocket
variation of substituents- aromatic substituents
variation of substituents around a ring system can alter the?
basicity
acidity of that part of the molecule
position of hydrogen groups on a ring
may increase potency