VSEPR theory
1/8
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
9 Terms
what is the octet rule
explain in terms of atomic orbits
the atoms in most compounds obey the octet rule meaning each atom forms enough bonds to have eight electrons in its outer shell. this makes sense with atomic orbitals as the valence shells of those atoms contain one s and three p orbitals, each of which can accept/hold 2 electrons for a total of eight.
4 exceptions to the octet rule
hydrogen has one 1s atomic orbital in its outer shell, so H atoms in molecules prefer an electron count of 2 rather than 8 (and He would be the same if it formed chemical compounds)
transition metals (groups 3-12) prefer 18 electrons as they have the five d orbitals in their valence shells as well.
boron is equally happy to have 6 or 8 electrons, as it has 3 valence electrons which form 3 covalent bonds, this means a 6-electron count is stable. as B is a small atom, BX3 molecules are quite crowded, so it is difficult but not impossible for a fourth bond to form.
heavier p-block elements including P, S, Cl and Br can form more bonds than they need for an octet giving them electron counts of 10, 12 or higher - these compounds are called hypervalent.
what is VSEPR theory
valence shell electron pair repulsion theory
used to predict the shapes of molecules based on the electron count at their central atom. electron pairs in a molecule arrange themselves to be as far apart as possible to minimise electrostatic repulsion between them, so the shape of a molecule can be worked out from how many pairs are at the central atom.
types of electrons recognised by VSEPR theory
electrons involved in covalent bonding (bonding pairs) and those that are not involved in covalent bonding (lone pairs). each covalent bond is counted as a single pair, regardless of whether it is single, double or triple
steps to work out VSEPR electron counts
count valence electrons at the central atom
count one electron from each bonded atom
add 1&2 them divide by two
decide how many bonding pairs and lone pairs there are
shape that the molecule shapes will be based on for 2, 3, 4, 5 and 6 pairs
2 - linear triangle
3 - triangle
4 - tetrahedron
5 - trigonal bipyramid
6 - octahedron
why do lone pairs repel more
bonding electron pairs experience the positive nuclear charge of two atoms’ nuclei, but lone pairs only experience one atom’s worth of positive charge. this means the electrostatic repulsion between the electrons in a lone pair is greater than in a bonding pair, which pushes them further apart from each other and makes the lone pair bigger. this in turn causes the pair as a whole to repel other pairs more
what is different about trigonal bipyramidal structures
in other shapes, all vertices are identical, but in a trigonal bipyramid the equatorial and axial sites are different from each other. the three equatorial sites are less compressed than the two axial sites. this means lone pairs prefer to occupy the equatorial sites (first).
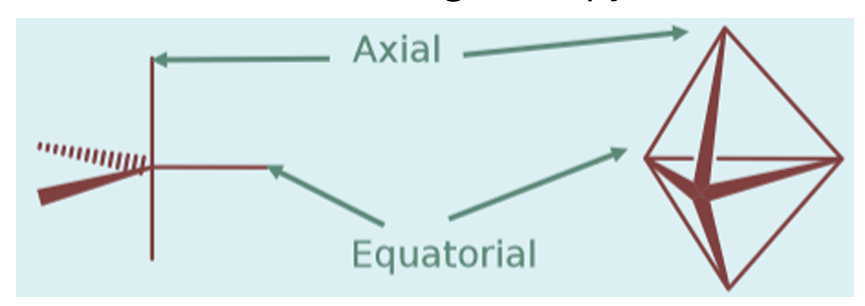
explain the less compressed equatorial sites of the trigonal bipyramid
the equatorial atom has more space around it, as it only has 2 nearest neighbours at 90° whereas the axial sites have 3 at 90°
