Biological Molecules (2c)
1/37
Earn XP
Description and Tags
Unit 2, Structure & Functions in Living Organisms: Part 1, c
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
38 Terms
Types of Biological Molecules
Categories:
Carbohydrates
Proteins
Lipids (fats and oils)
All contain carbon, so are called organic molecules
Molecules and Chemical Elements
Carbohydrates: Carbon, Hydrogen, Oxygen
Proteins: Carbon, Hydrogen, Nitrogen, Oxygen, additional elements including Sulfur
Lipids: Carbon, Hydrogen, Oxygen
Structure of Carbohydrates
Contain C, H, O
made of starch and glycogen
can be small, simple sugars or larger, more complex molecules
monosaccharide: simple sugar, eg, glucose (C6H12O6)
disaccharide: two monosaccharides joined together, eg, maltose = 2 glucose joined together
large polysaccharides: many monosaccharides joined together, eg, starch, glycogen or cellulose = many simple glucose molecules joined together
polysaccharides: insoluble = storage molecules
Structure of Lipids
large molecules made of smaller basic units like glycerol and 3 fatty acids
solid at room temperature (fats) or liquid at room temperature (oils)
Storage of Glucose: Plants vs Animals
plants: starch
animals: glycogen
Structure of Proteins
made of smaller molecules called amino acids
when these are joined, proteins are formed
can be arranged in any order
specific amino acid gives protein its shape
shape determines function
ex: keratin, haemoglobin
Molecule vs Monomer
Carbohydrate : glucose
Lipids: glycerol and fatty acids
Protein: amino acids
Food Samples can be investigated for:
glucose
starch
fat
protein
Preparing a Sample
break up food with a mortar and pestle
dilute with distilled water
stir with a glass rod to mix
filter through filter paper and collect solution
proceed with food tests
OR
use powdered food
Preparing a Sample: Precautions
eye protection
wash splashes quickly
don’t taste food substances
avoid spilling hot water
Test for Glucose
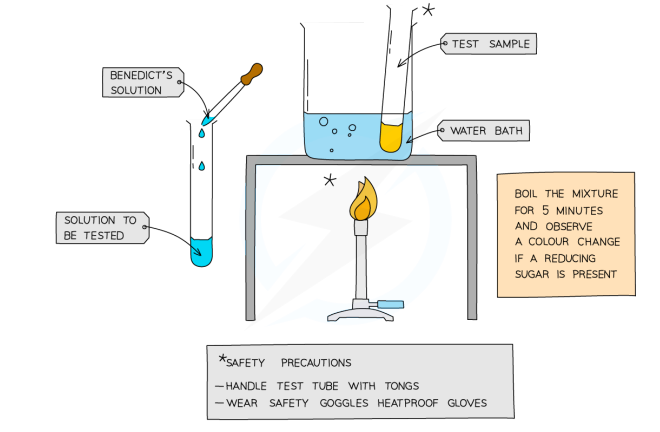
Benedict’s Test
add a few drops of bright blue reagent to the sample
heat in very hot water for 5 mins
observe if there’s a colour change
positive result: blue → green → yellow → orange → brick red (depending on conc.)
Benedict’s Solution: Safety Precautions
wear safety goggles
switch off bunsen burner after water is almost boiling / use water bath
Test for Starch
Iodine Test
Iodine solution is yellow-brown
add several drops to the sample
positive result: yellow-brown → blue-black
Protein Test
Biuret Test
Biuret solution is blue
positive test: blue → lilac
Lipids (Fats) Test
Ethanol Emulsion Test
mix food sample with 4cm3 of ethanol
ethanol is a clear and colourless liquid
place the bung firmly and shake vigorously
allow the sample time to settle
strain the solution into another test tube
add the ethanol solution to an equal volume of cold distilled water (4cm3)
positive result: cloudy emulsion
Food Tests Hazards
Biuret solution contains copper (II) sulfate, which is dangerous if it gets into eyes → always wear goggles
Iodine solution irritates eyes
NaOH in Biuret solution is corrosive → wash hands immediately if any chemicals get onto skin
Ethanol is highly flammable → keep away from Bunsen burner
Bunsen burner is an open flame → keep off when not in use
Enzymes as Biological Catalysts
enzymes are proteins that speed up the rate of reaction without being used up in it
called biological because made in living cells
necessary because they maintain reaction speeds of all metabolic reactions at a rate that can sustain life
if we didn’t produce enzymes, digestion would take 2-3 weeks, with enzymes it takes 4 hours
often the products of one reaction are the reactants of another and so on
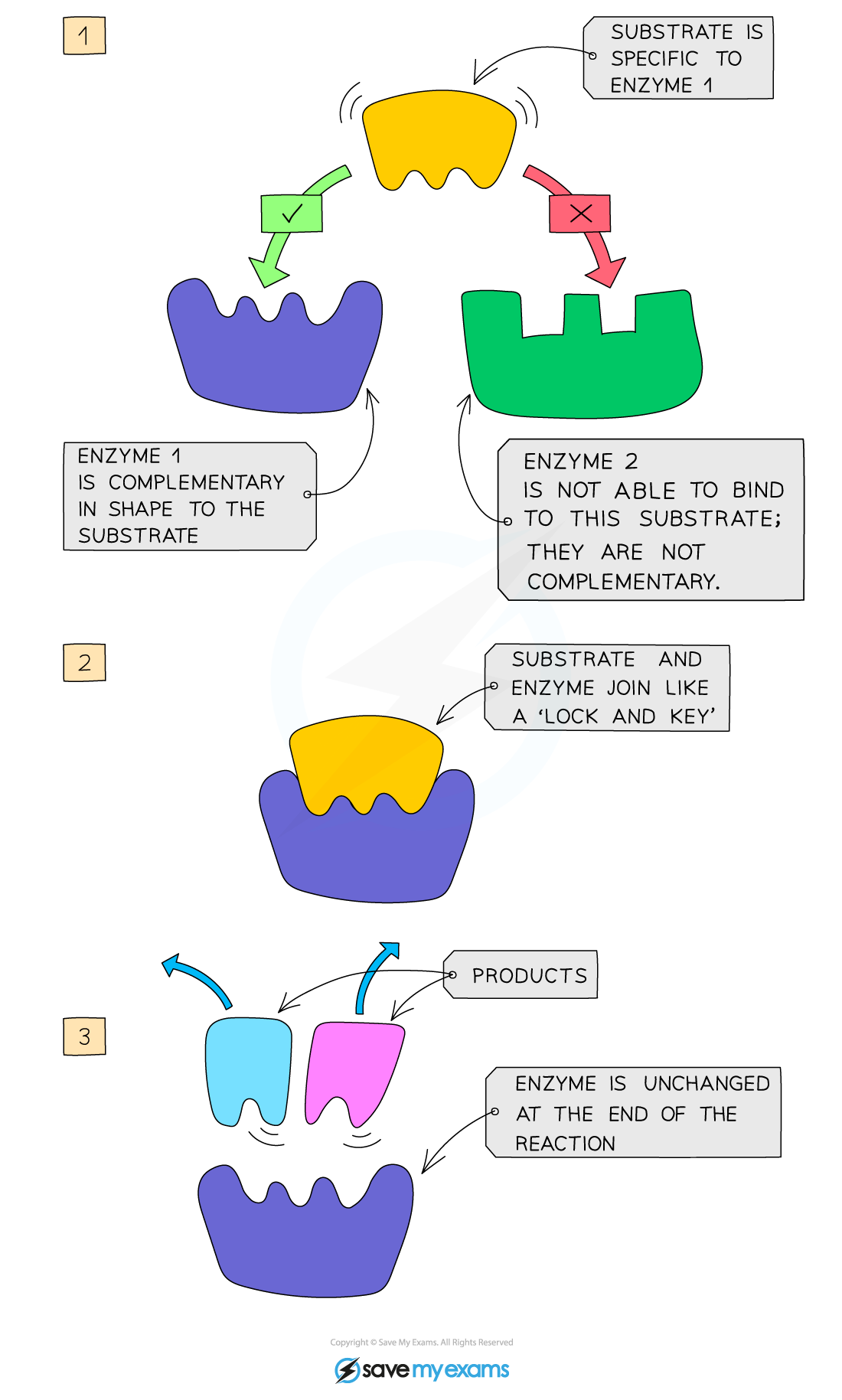
Enzyme Action Mechanism
an enzyme’s active site is a complementary shape to the substrate, so enzymes are specific to one particular substrate
when the substrate moves into the active site, they become known as an enzyme-substrate complex
after reaction, products leave the active site as they no longer fit it
the enzyme takes up another substrate
Enzyme Process
enzymes and substrates move randomly in solution
when an enzyme and complementary substrate collide, an enzyme-substrate complex forms and a reaction occurs
products are formed and released from the active site. enzyme is unchanged and can catalyse further reactions
Amylase digests __________ into _________
starch, maltose
Starch
large biological molecule formed from many glucose molecules joined together
Maltose
molecule formed from two glucose molecules joined together
Enzymes and Temperature Practical: Safety
Iodine and Amylase can irritate eyes → wear safety goggles
Amylase irritates skin → wear gloves
Avoid burns from very hot water → handle equipment with tongs
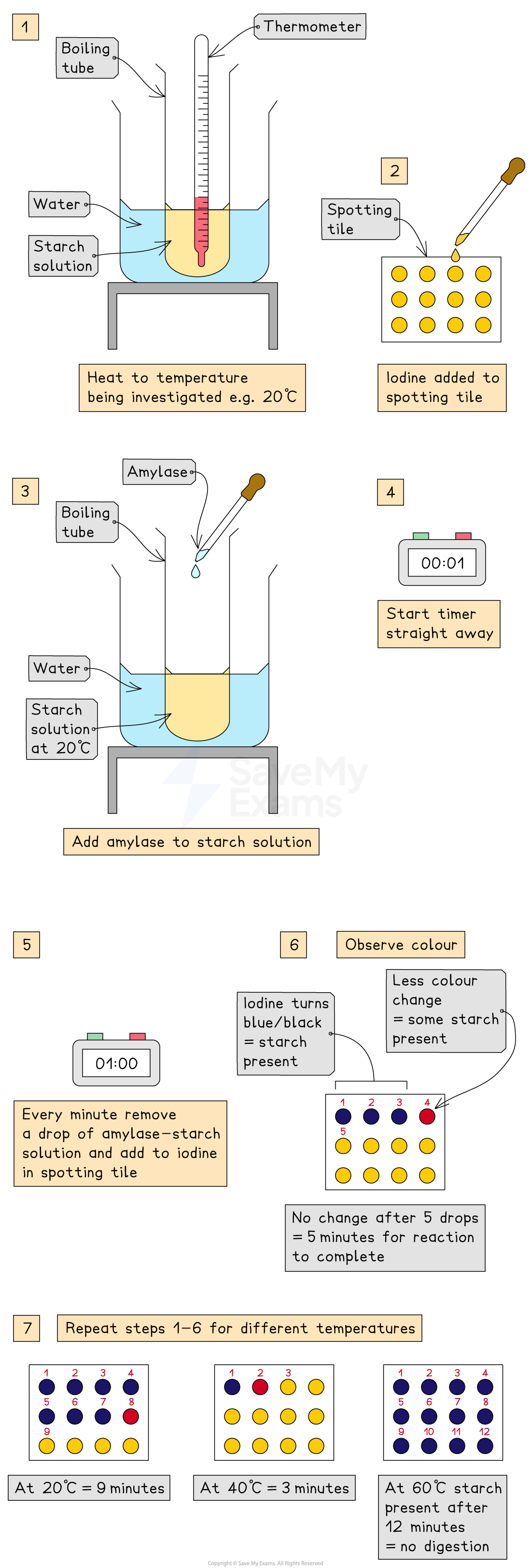
Enzymes and Temperature Practical: Method
add 5cm3 of starch solution to test tube and heat in a 20oC water bath
add a drop of Iodine solution to each well of the spotting tile
use a syringe to transfer 2cm3 amylase to starch solution and mix.
start timer
after one minute, transfer one drop of solution to the spotting tile’s first well
repeat every minute until iodine stops changing colour → amylase has broken down all the starch by now
record time taken by counting to the first well where there is no colour change in Iodine
repeat at same temperature at least twice
repeat for a range of temperatures (eg. 30-60oC)
Enzymes and Temperature Practical: Limitations
temperature may not be constant/stable/precise since Bunsen burner is used → store starch and amylase solutions in a water bath for about 10 mins before being mixed
determining when starch has been digested relies on visual observation, which is subjective → use colorimeter (measures intensity of colour by shining light through it and measuring how much light passe through)
Enzymes and Temperature Practical: CORMMS
C: changing the temperature
O: irrelevant
R: repeat several times for each temperature to increase reliability
M1: measure the time taken for iodine to stop turning blue-black
M2: by counting the number of spotting tiles
S: control the concentration and volume of starch solution, iodine, and amylase used
Enzymes and Temperature Practical: Optimum Temperature
iodine stops turning blue-black the fastest
enzyme is working at its fastest rate and has digested all the starch in the solution
Enzymes and Temperature Practical: Colder Temperature
iodine takes longer time to stop turning blue-black
Amylase is working slowly due to low kinetic energy and fewer collisions between Amylase and starch
Enzymes and Temperature Practical: Hotter Temperatures
iodine turned blue-black throughout investigation
Amylase denatures and can no longer bind with starch or break it down
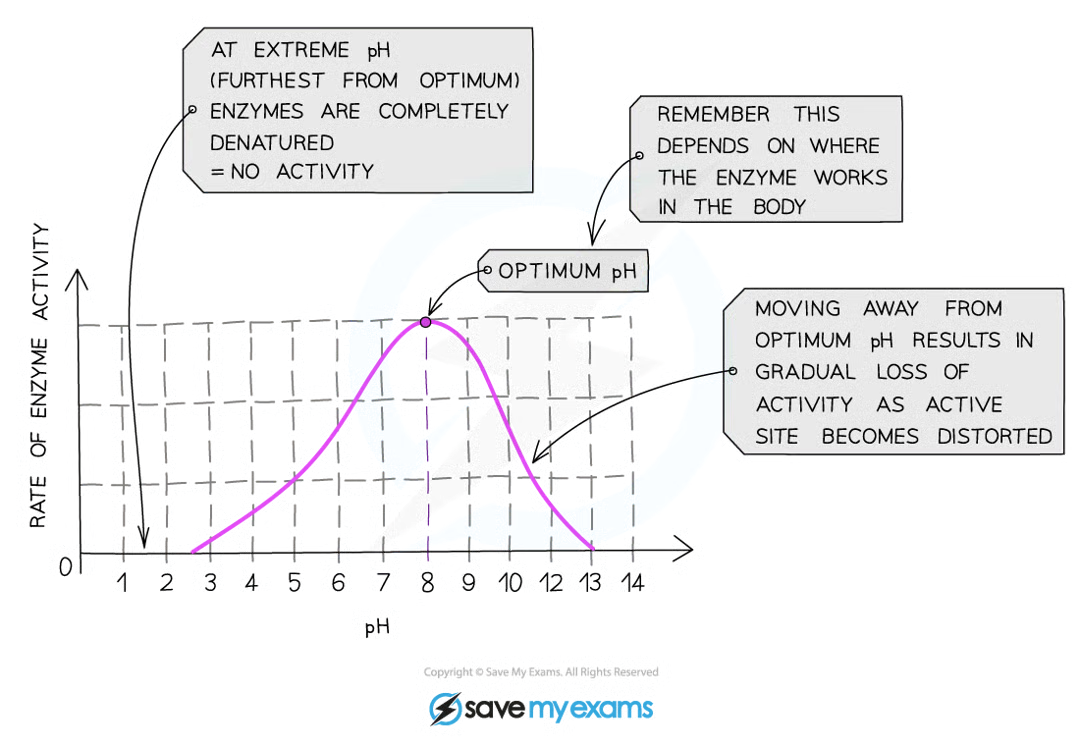
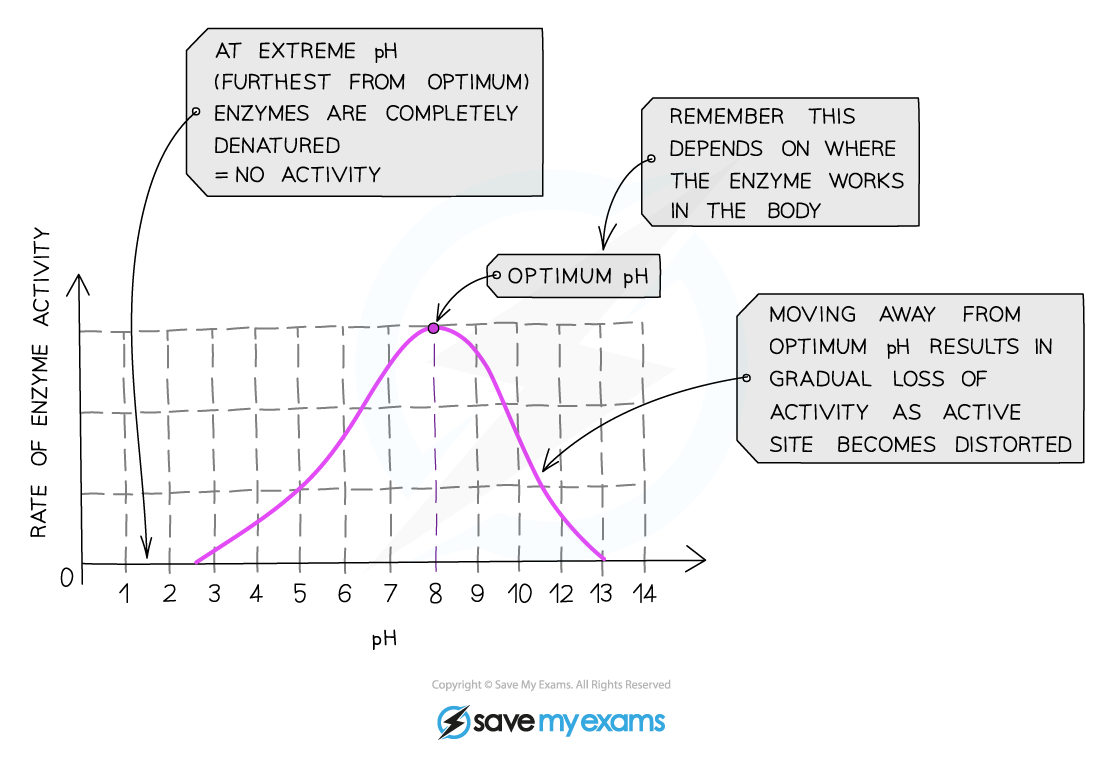
Optimum pH for most enzymes is _____________
7
ph for enzymes in acidic conditions like stomach
low, pH 2
pH for enzymes in alkaline conditions like duodenum
high, pH 9
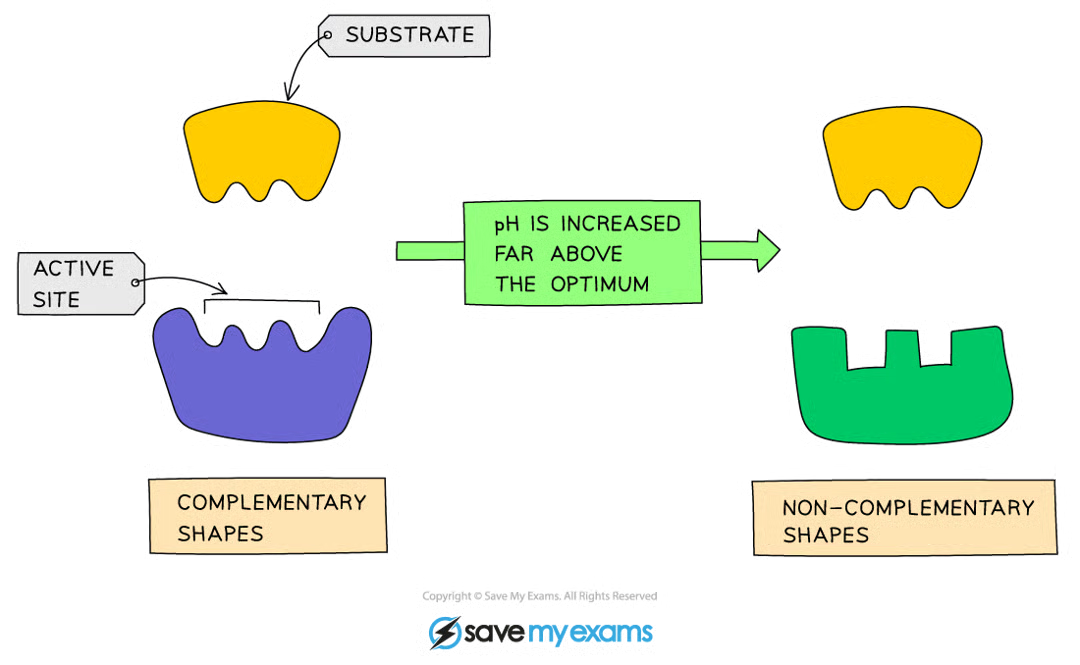
Effect of pH being too high/too low
bonds holding amino acid chain together to make up protein will be destroyed
active site shape will change, substrate will no longer fit
moving too far from optimum pH → enzyme denatures, activity stops
Effect of pH on Enzymes Practical: Apparatus
Spotting tile
Measuring cylinder
Test Tube
Syringe
Pipette
Stopwatch
Buffer solutions at different pH levels
Iodine
Starch solution
Amylase solution
Effect of pH on Enzymes Practical: Safety
both Iodine and Amylase cause irritation- wear goggles
Amylase causes irritation- wear gloves
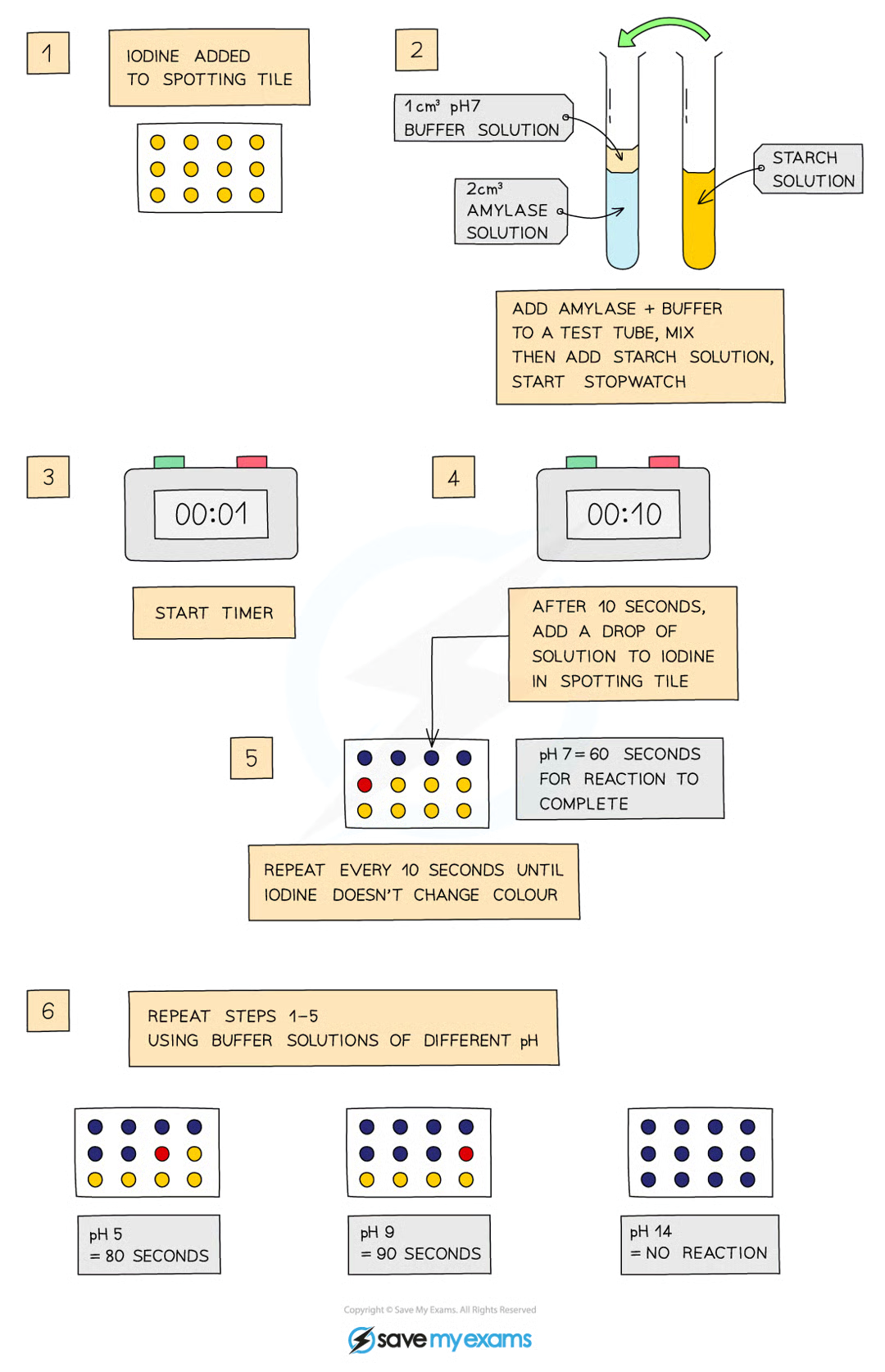
Effect of pH on Enzymes Practical: Method
add drops of I to each well of the spotting tile
use syringe to place 2cc of amylase into test tube
add 1cc of buffer (pH 3) to test tube with syringe
use another test tube to add 2cc of starch solution to amylase and buffer solution, start stopwatch while mixing with a pipette
every 10s, transfer a drop to a well of I solution (should turn blue-black)
repeat until I solution stops turning blue-black (amylase has broken down all the starch)
record time taken by counting wells until the solution stopped changing colour
repeat at different pH values
Effect of pH on Enzymes Practical: Results and Analysis
Amylase is an enzyme that breaks down starch
when I solution remains orange-brown, all the starch has been digested
at optimum pH, colour stopped changing in the shortest time
because enzyme is working at its fastest rate and has digested all the starch
at higher or lower pH’s (above/below the optimum): I took longer to stop changing colour or continued to change colour throughout
because on either side of the optimum pH, enzymes are denaturing and are unable to bind with the starch or break it down
Effect of pH on Enzymes Practical: CORMMS
C - change the pH of the environment
O - same concentration of enzyme
R - repeat the investigation several times to ensure reliability
M1 - measure the time taken by counting the number of wells for
M2 - the iodine to stop turning blue-black (1 well = 10 seconds)
S -control the temperature and volume of the amylase, iodine and starch solution used in the investigation