CHEM 261 - Substitution and Elimination Theory
1/27
Earn XP
Description and Tags
I didn't think this would be as important as the mechanism itself, but it is (unfortunately)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
28 Terms
Nucleophile
Is any negative ion or neutral molecule which possesses a long-pair of electrons and are attracted to a positive center.
Is also a base (lewis base).
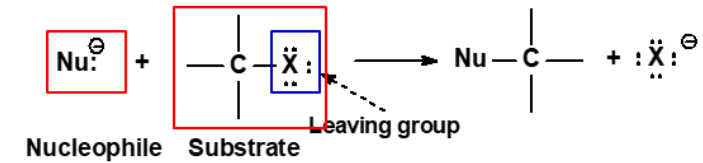
Substrate
The alkyl halide that is being attacked by the nucleophile.
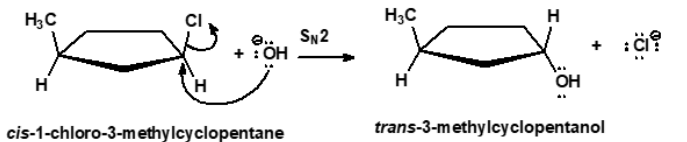
Inversion of Configuration
The process in which the configuration of a chiral center is reversed during a substitution (SN2) reaction, resulting in a product with opposite stereochemistry.
Substitution (Bimolecular) SN2
Reaction involves a backside attack by a nucleophile on the sp3 carbon.
It is a concerted reaction - bond making and bond breaking at the same time.
A transition state is formed in the reaction - the nucleophile, sp3 and the leaving group lie in a straight line.
The sp3 carbon becomes sp2 hybridized and the three substituents all lie on the same plane.
A good leaving group is a weak base.
Reactivity towards SN2
Methyl > 1° > 2° > 3°.
Reaction is also sensitive to steric effect - too much crowding slows the reaction and too much steric hindrance can stop the reaction altogether.
Alters rate of reaction.
Leaving Group
A atom or group that can be easily displaced in reaction.
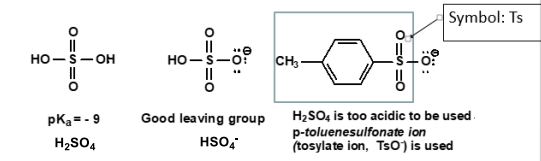
The weaker the basicity, the better the leaving group.
Fluorine is a poor LG, whereas I is a good LG.
Other good LG’s include H₂SO₄, HSO₄⁻, TsO⁻ (used when H₂SO₄ is too acidic).
Nucleophilicity
Usually parallels basicity - a strong base is usually a strong nucleophile.
A negative charged nucleophile is always stronger than its conjugate acid.
Across a period, nucleophilicity (basicity) decreases from left to right.
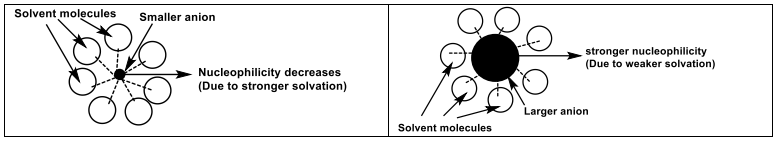
Basicity normally decreases with atomic size (down the table). But in a polar solvent, nucleophilicity increases down the periodic table (large anion would become a strong nucleophile).
Decreases when there’s a bulky group present.
Solvation
Intermolecular interactions (h-bonds, dipole-dipole, ion-dipole) between the solvent molecules and the reagents/transition states.
When the solvent is water, the solvation is referred to as hydration.
The intermolecular forces (solvation) is largest for smaller anions.
Solvation of the anions decreases the nucleophilicity.
Protic Solvent
A solvent which possesses hydrogen attached to electronegative elements.
i.e. water and methanol.
They form hydrogen bonds and nucleophiles.
Protic solvents solvates anion.
Nucleophilicity is therefore decreased - energy is needed to strip off the solvent molecules.
The strong interaction between methanol (a polar protic solvent) and anion lowers its reactivity.
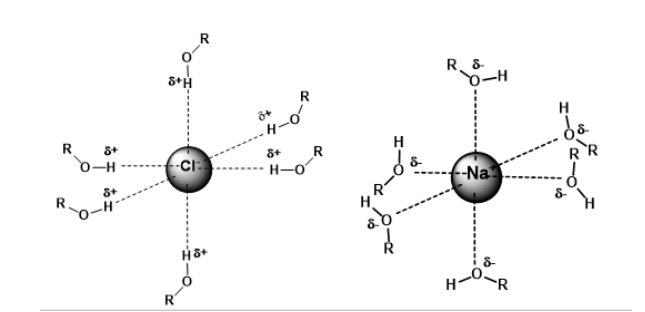
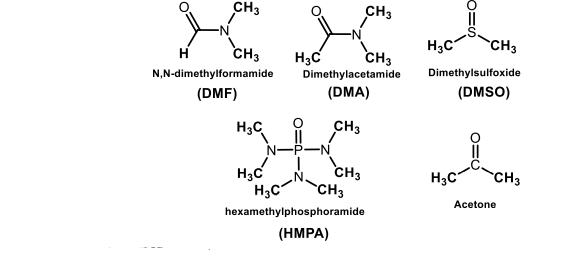
Aprotic Solvent
Is a solvent whose molecules do not possess hydrogen attached to electronegative elements.
Some common polar aprotic solvents are N,N-dimethylformamide (DMF) and dimethyl sulfoxide (DMSO).
The type of solvent greatly affects the rate of reaction.
Aprotic solvents solvates cation only, leaving the anion “naked.”
Nucleophilicity of anion is therefore increased.
Solvation of the Anion
The strong interaction between methanol (a polar protic solvent) and anion lowers its reactivity.
Factors Affecting SN2 Reaction
The substrate: CH₃ > 1° > 2° > 3°.
The nucleophile: Strong (usually strong bases).
The leaving group: Weak bases.
The solvent: Aprotic.
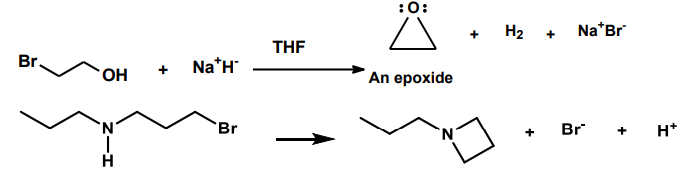
Intramolecular Substitution Reactions
A functional group within a molecule is replaced by another atom or group within the same molecule, leading to the formation of new cyclic structures.
Substitution Unimolecular (SN1)
Tertiary alkyl halides undergo SN1 substitution. Enhanced by polar protic solvents.
Rate = k[alkyl halide]; nucleophile strength doesn't affect rate, so weak nucleophiles can participate.
The slow, rate-determining step forms a carbocation.
A weak nucleophile then rapidly attacks the carbocation from either side (no steric hindrance).
Finally, the solvent is deprotonated and attaches to the substrate.
Hyperconjugation
Delocalization of electrons from a bonding orbital to an adjacent unfilled orbital.
Stabilizes the transition state; the more stable the carbocation, the lower its activation energy.
As a result, it increases the reaction rate.
Summary of SN1 and SN2 Reactions
Factors | SN1 | SN2 |
Alkyl Groups | CH3 and 1° | 3° |
Leaving Groups | Good leaving groups | Good leaving groups |
Nucleophile | Strong Nucleophile | Weak Nucleophile |
Solvents | Polar aprotic solvent | Polar protic solvent |
Elimination Reaction
The loss of two atoms or groups from a group from a substrate to the formation of a π bond.
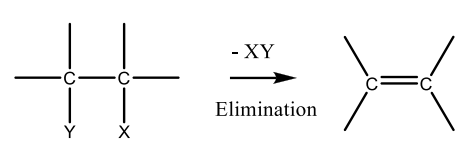
Dehydrohalogenation
A reaction in which a hydrogen atom and a halogen atom are removed from adjacent atoms in a molecule, forming (usually) an alkene or an alkyne.
Bases commonly used for this reaction include KOH and a conjugate base of alcohol (i.e. a sodium salt).

Dehydration
The chemical reactions in which a water molecule is eliminated from the reactant molecule.
Can result in a alkene.
Orientation of Carbon
The carbon atom bonded to the leaving group X is called the α-carbon.
The adjacent carbon atom is called the β-carbon.
The next carbon atom in the chain is termed the γ-carbon.
Unimolecular Elimination (E1)
A two step-reaction. The first step entails the formation of a carbocation.
The second step is the loss of a hydrogen on a β-carbon.
Since the rate-determining step is the same for both SN1 and E1 by the formation of a carbocation, an E1 reaction always results in the formation of a SN1 side product.
There are major and minor products though - elimination reactions favour heat.
The more substituted alkene is always the major product.
Rate = k[RX]/
![<ul><li><p>A two step-reaction. The first step entails the formation of a carbocation.</p></li><li><p>The second step is the loss of a hydrogen on a β-carbon.</p></li><li><p>Since the rate-determining step is the same for both S<sub>N</sub>1 and E1 by the formation of a carbocation, an E1 reaction always results in the formation of a S<sub>N</sub>1 side product.</p><ul><li><p>There are major and minor products though - elimination reactions favour heat.</p></li><li><p>The more substituted alkene is always the major product.</p></li></ul></li><li><p>Rate = k[RX]/</p></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/18c1f3d9-7f43-4d8c-a190-99c229490989.png)
Bimolecular Elimination (E2)
E2 is a one-step, concerted reaction with rate = k[RX][Base].
A strong base drives elimination with no substitution by-products.
Simultaneously:Base forms ROH.
β-H is removed (H–C bond breaks).
C=C π bond forms.
C–Br bond breaks (leaving group exits).
Zaitsev’s Rule
if more than alkene product is possible, the most stable alkene (with most substituents) is formed as the major product.
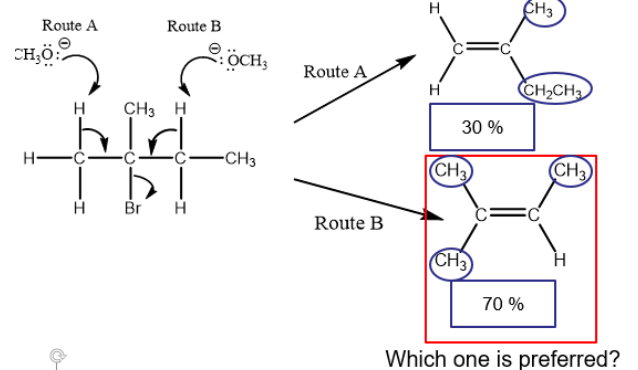
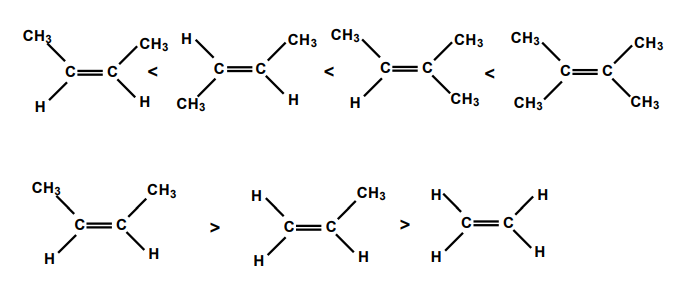
Stability of Alkenes
The preferred product is the alkene with the breater number of alkyl groups attached to the carbon atoms with the double bond.
Trans-alkene is more stable than the cis-alkene.
Condition for a π Bond Formation
The p-orbitals must be on the same plane for effective side-to-side overlapping.
Overlapping is not possible if the p-orbitals are not on the same plane.
Syn-Periplanar Elimination
The spatial arrangement in which atoms or groups involved in a chemical reaction, specifically in an E2 elimination reaction, are on the same side and in the same plane.
Stereochemistry for E2 Reaction
E2 mechanism is concerted.
β-hydrogen, the leaving group must lie in the same plane for reaction for that a π bond can be formed effectively.
The elimination reaction takes place when β-hydrogen and the leaving group are anti (anti-elimination) to each other in the Newman projection.
If the β-hydrogen and the leaving group are not on the same plane and anti to each other, E2 mechanism is not possible.
Elimination Vs. Substitution Table
Types of Alkyl Halides | Weak Nucleophile/Base | Strong Base Nucleophiles | Strong and Bulky Base Nucleophiles |
Methyl | No reaction. | SN2 | SN2 |
1° | No reaction. | SN2 | E2 |
2° | E1 and SN1 | SN2 | E2 |
3° | E1 and SN1 | E1 and SN1 | E2 |