AP Bio Unit 1 Test Answers
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
A protein is a polymer made up of which kind of monomers?
amino acids
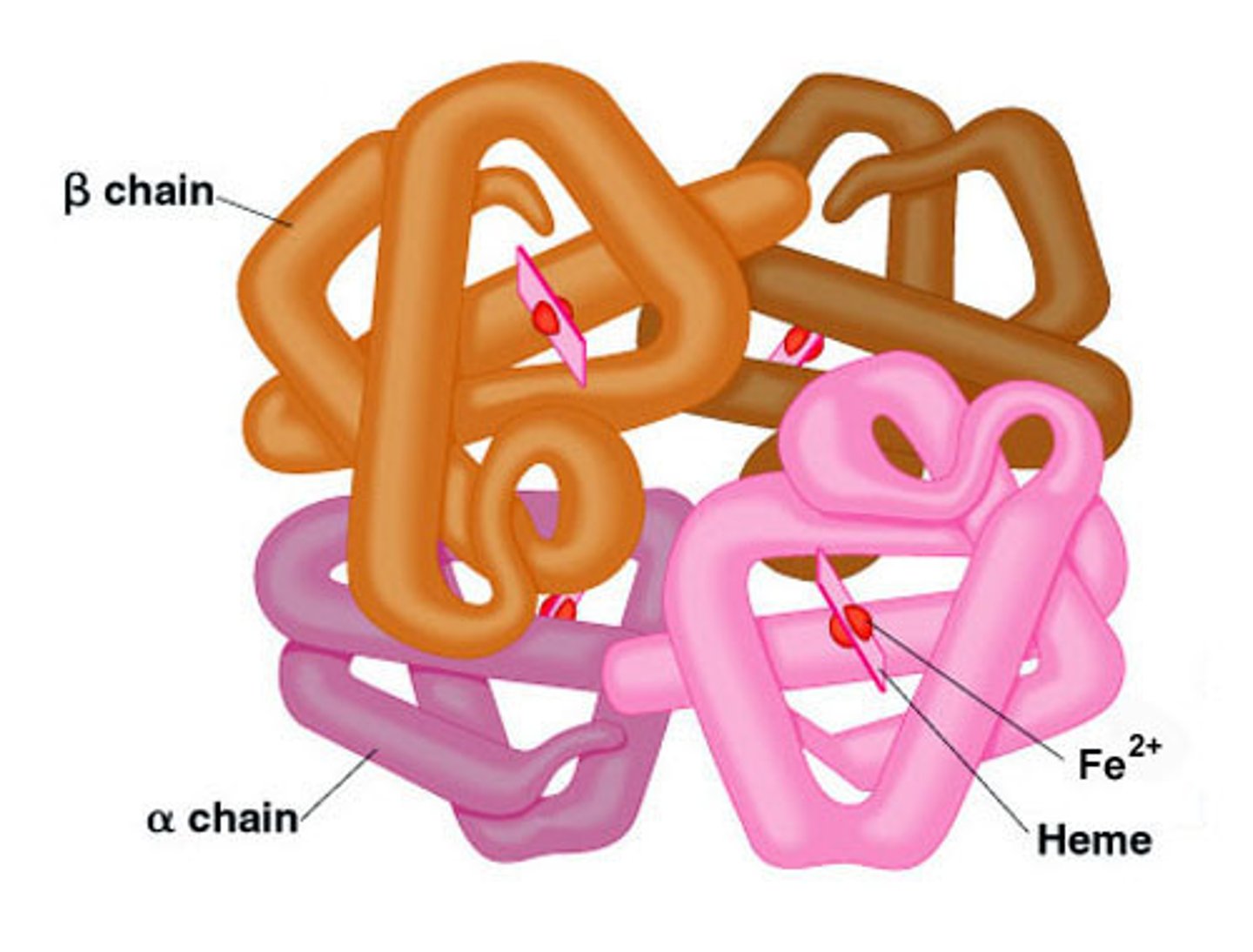
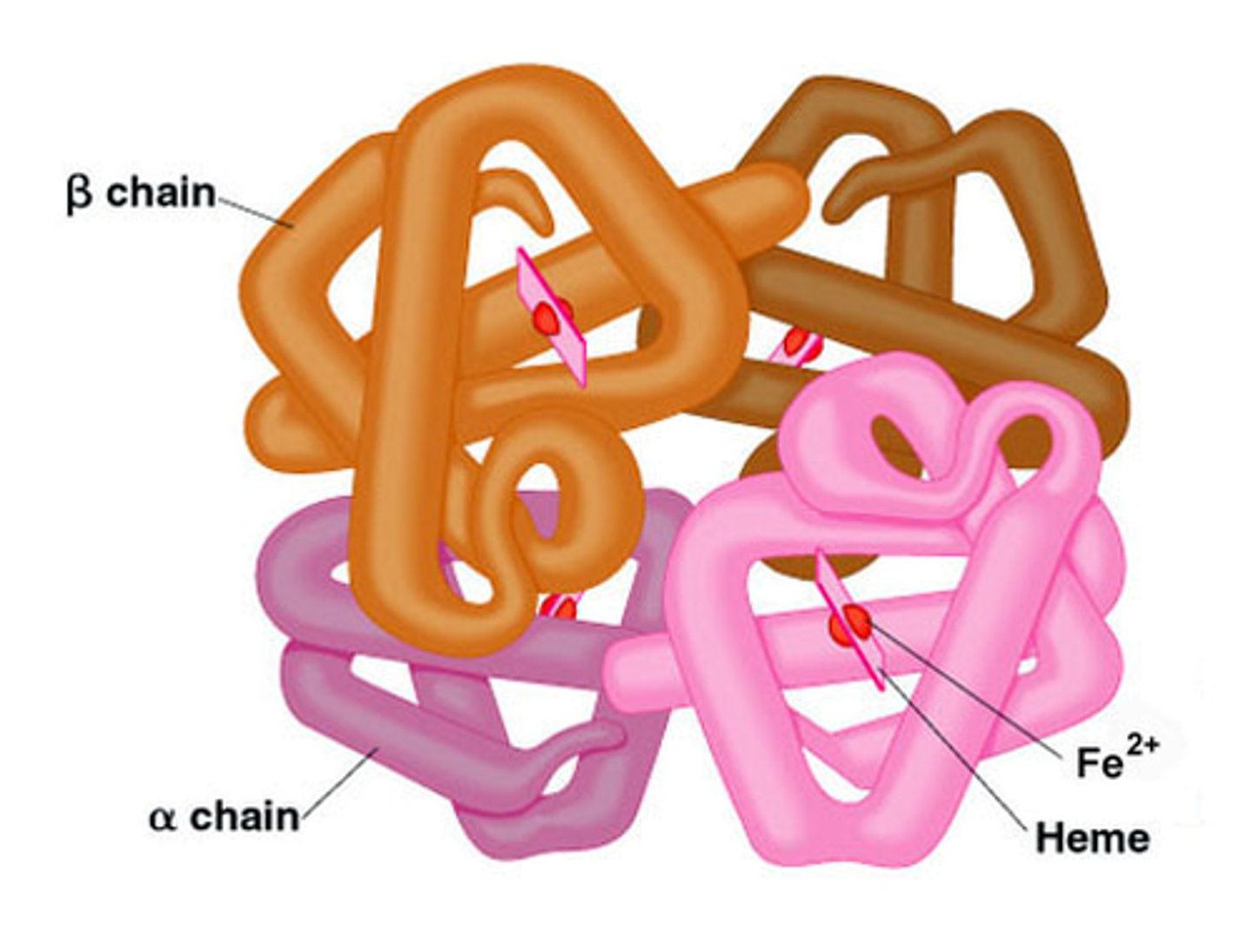
The diagram above shows the final form of the protein hemoglobin. The making of this protein depended on the presence of chaperone proteins. Predict the consequence if chaperone proteins had NOT been present.
hemoglobin would not have folded up correctly
The breakfast dishes have dried yellow egg on them when you return in the evening. The dried yellow egg remains after you dunk the dirty dishes in water. After soaking awhile, the complex of various egg yolk molecules easily "washes off." What has happened?
Soaking in water eventually resulted in hydrolysis reactions where water broke these bonds.
The hemoglobin molecule above displays the __________ level of protein structure.
quaternary
One of the main differences between the two polysaccharides cellulose and starch is
the functions they perform in plants.
A peptide bond is found in which type of biological molecule?
protein
Hydrogen bonds that form α helices and β-pleated sheets are the primary interaction in which level of protein structure?
secondary
Below freezing and above boiling, cells are unable to function as "liquid machinery." However, most organisms' cells exhibit limited functioning at extreme temperatures. At the molecular level in different organisms, a cells' ability to withstand temperature extremes, etc., is most closely related to variation in
enzyme activity and protein denaturation
Which polymer consists of a sugar, a phosphate, and a base?
nucleic acid
Which of the following correctly relates the biomolecule with its function?
Chitin; structural component in fungi cells
Which statement is true about RNA?
One of the bases from DNA is replaced by uracil.
A chemist has analyzed the chemical makeup of three compounds and determined the relative amounts of the elements in each. The results are summarized in the table below.
According to the chemist's results, Compound 1 is C₆H₁₂O₆
Carbohydrate
Both glyceraldehyde and dihydroxyacetone have the molecular formula C3H6O3. However, the double-bonded oxygen in glyceraldehyde is attached to the end carbon while the dihydroxyacetone molecule has the double-bonded oxygen bonded to the middle carbon.
These molecules are isomers with different structural arrangements of the same atoms
How do the lipids of the cell membrane and the lipids found in butter and vegetable oil differ?
the number of fatty acids attached to the glycerol molecule
Organic molecules are those that contain at least
carbon and hydrogen.
A polysaccharide is a polymer made up of which kind of monomers?
simple sugars
The reactivity of an organic molecule is primarily dependent upon ____________ of the molecule.
the attached functional groups
The structural polysaccharides found in different kingdoms are all variations of cellulose. Each kingdom has unique modifications to cellulose. Which kingdom is correctly paired with its structural polysaccharide?
Fungi; chitin
Which carbohydrate is used in the liver for energy storage?
glycogen
Fats, oils, and steroids are
lipids.
What subunits makeup a lipid macromolecule?
Fatty acids and glycerol
The two molecules on the left side of the above equation are
amino acids

If the reaction were to proceed from left to right, this would be an example of what type of reaction?
dehydration

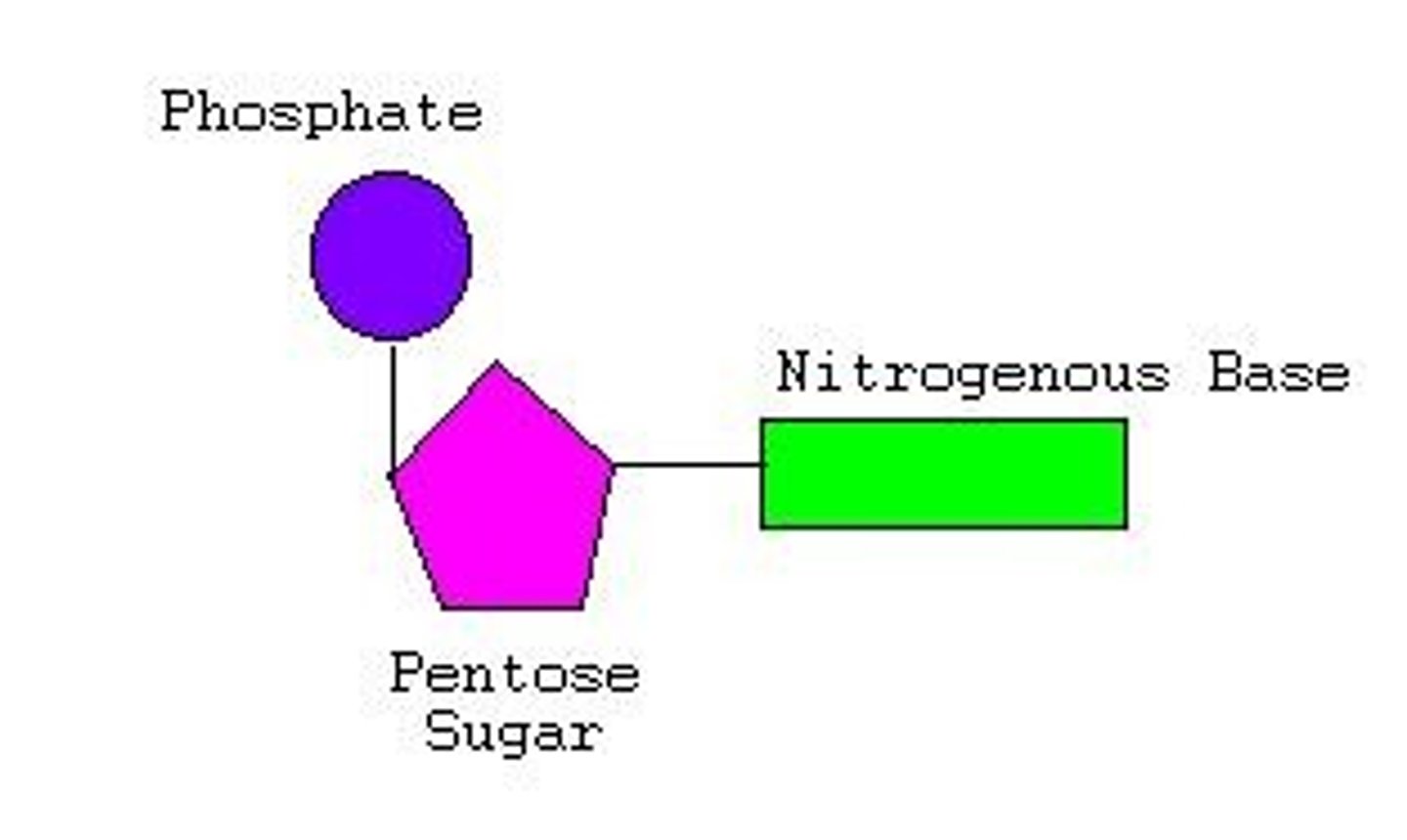
The molecule on the right above represents the basic structure for which of the following?
nucleotide
Which of the following set of words best completes the statement:A(n)_____ fat will have _____ bonds between the carbon atoms of the fatty acid and will be a ____ at room temperature.
unsaturated; double; liquid
Which of the following atoms has four electrons available for covalent bonding, and can form rings, long chains, and double bonds, making it the building block of the most versatile complex biological molecules?
Carbon
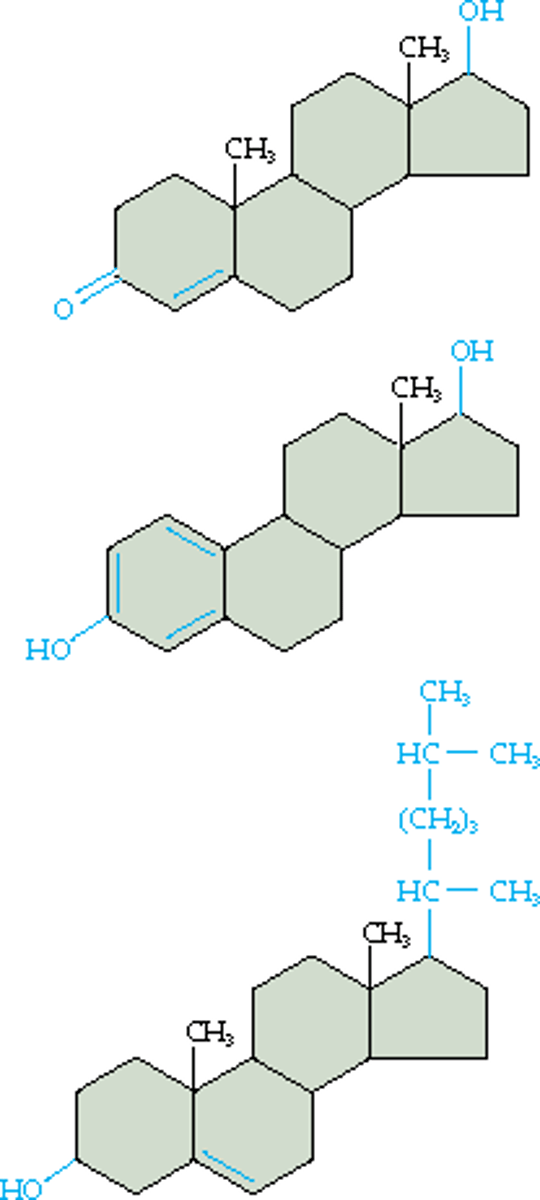
The structural diagrams above are examples of
steroids.
How would this molecule be altered to produce adenosine monophosphate (AMP)?
Change the C (cytosine) to A (adenine)
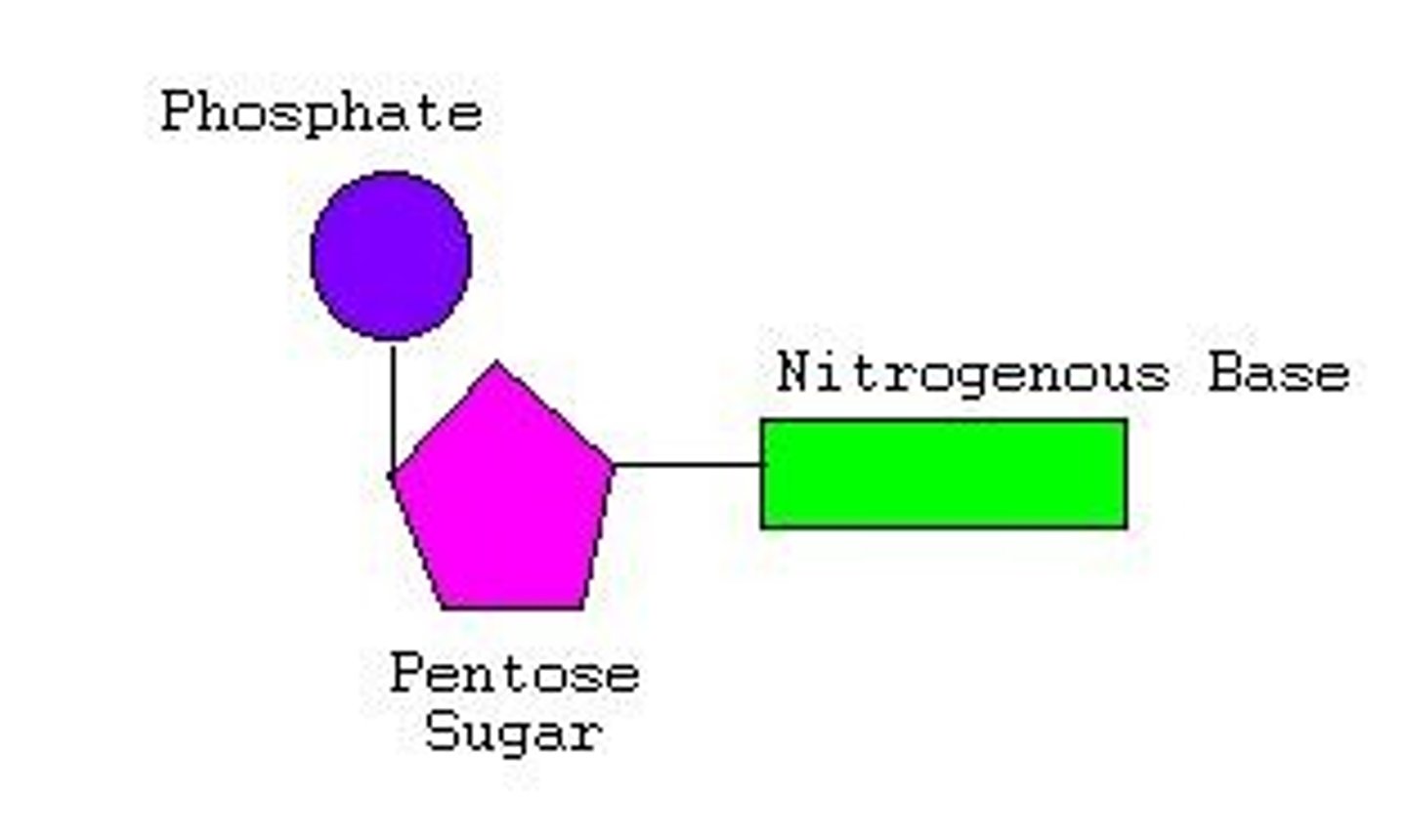
Ribonucleic acid (RNA) and deoxyribonucleic acid (DNA) differ structurally in that
DNA contains deoxyribose, and RNA does not.
If 10 amino acids are joined together by dehydration synthesis, how many molecules of water are liberated during the chemical reaction?
Multiple choice question.
9