Exam 3 - Cell Structure Part 1
0.0(0)
0.0(0)
Card Sorting
1/137
Earn XP
Description and Tags
Last updated 12:49 AM on 11/2/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
138 Terms
1
New cards
Endoplasmic Reticulum
comprises a network of membrane that penetrates much of the cytoplasm (~50% of total membrane); highly dynamic; discovered in 1945 by Keith Porter, Albert Claude, Ernest Fullam
2
New cards
Smooth Endoplasmic Reticulum (SER)
short tubules
varies greatly in size in different cell types; will expand when needed
varies greatly in size in different cell types; will expand when needed
3
New cards
Rough Endoplasmic Reticulum (RER)
rough appearance due to the presence of ribosomes
flattened sacks
composition of the luminal or cisternal space is different from the surrounding cytosolic space
cytoplasmic entry point for proteins into vesicular trafficking system called biosynthetic pathway
protein synthesis on membrane bound-ribosomes vs free ribosomes
protein modifications
flattened sacks
composition of the luminal or cisternal space is different from the surrounding cytosolic space
cytoplasmic entry point for proteins into vesicular trafficking system called biosynthetic pathway
protein synthesis on membrane bound-ribosomes vs free ribosomes
protein modifications
4
New cards
cell biology
study of the structures that make up a cell, how these structures function, form, and are maintained
5
New cards
Compartmentalization
Differences between prokaryotic and eukaryotic cells;
cellular compartment is (usually) a membrane encapsulated structure; means that proteins must be targeted to specific locations in the cell
cellular compartment is (usually) a membrane encapsulated structure; means that proteins must be targeted to specific locations in the cell
6
New cards
Eukaryotic cells
cell that is highly compartmentalized;
A way to optimize cell behaviors and chemical reactions
- concentrate and isolate reactants
- optimize environment for reactions
A way to optimize cell behaviors and chemical reactions
- concentrate and isolate reactants
- optimize environment for reactions
7
New cards
routing possibilities for proteins
cytoplasm
nucleus
mitochondria
endoplasmic reticulum
nucleus
mitochondria
endoplasmic reticulum
8
New cards
sorting signals
amino acid sequences within proteins that direct protein localization and transport;
oligosaccharides attached to amino acids; Amino acids have different properties
oligosaccharides attached to amino acids; Amino acids have different properties
9
New cards
proteins
_______________can passively diffuse to correct location in cell, or be transported within the cell
10
New cards
type of protein transport
gated transport
Translocator-based transport
Vesicular transport
Translocator-based transport
Vesicular transport
11
New cards
gated transport
controlled opening of pore complexes; (Ex., selectivity of nuclear pore function in the nuclear envelope); folded proteins can go through, both passive and active transport
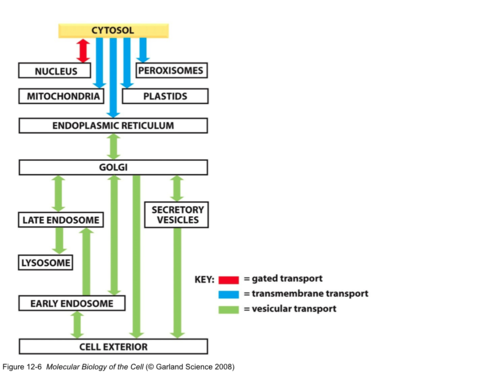
12
New cards
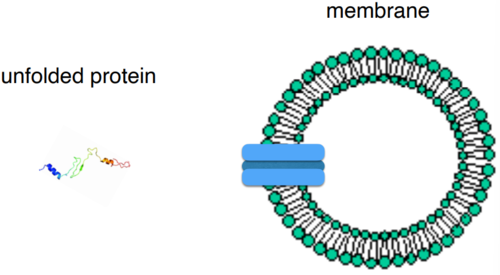
Translocator-based transport
transmembrane protein translocators transfer protein from one side of membrane to another. Protein must be unfolded to pass through.
13
New cards
Vesicular transport
often small spherical membrane packages that ferry proteins from one compartment to another
Requires:
-selection of cargo
-formation of a vesicle (budding)
-fusion of a vesicle with a target compartment
Requires:
-selection of cargo
-formation of a vesicle (budding)
-fusion of a vesicle with a target compartment
14
New cards
How do we identify what the signal sequence is for a given protein?
Molecular Biology approach
Biochemical approach
Genetic approach
Biochemical approach
Genetic approach
15
New cards
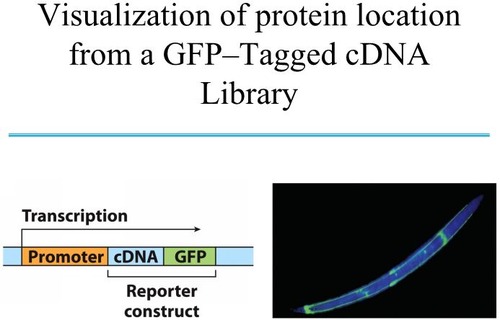
Molecular Biology approach
Take bits of transported protein and fuse or "clone" amino acid sequence to a reporter protein and check distribution.A classic reporter gene... GFP
16
New cards
Biochemical approach
Direct purification of signal sequence
17
New cards
Genetic approach
Break (mutate) specific parts of protein to identify sequence for localization
18
New cards
Electron microscopy
First electron microscope invented in 1931
By the 1940s, cells were being stained with osmium and imaged with electron microscope
By the 1940s, cells were being stained with osmium and imaged with electron microscope
19
New cards
Why use electron microscopy?
Resolution of optical instruments is limited by the diffraction limit ~1/2 wavelength of light Electrons wavelength ~100,000x smaller than visible light
20
New cards
contiguous
ER and nuclear envelope are _________________.
21
New cards
ER subcompartments
Smooth ER
Rough ER
Rough ER
22
New cards
Extracellular
outside the cell
23
New cards
cytoplasmic
Pertaining to cell matter (cytoplasm)
24
New cards
lumenal
Inside. Interior open space or cavity of a tubular organ (ER); becomes into cytoplasm once it leaves an organism
25
New cards
Smooth ER
functions in
- Ca2+ storage
- production of lipids, phospholipids and steroids (testestorne)
- detoxifying enzymes
- Ca2+ storage
- production of lipids, phospholipids and steroids (testestorne)
- detoxifying enzymes
26
New cards
proteins
_______________ need to be "targeted" to the right compartment
27
New cards
targeting sequence
_________________is part of protein itself
28
New cards
Membrane-bound ribosomes vs free ribosomes
no difference in the ribosomes per se
site of protein synthesis determined by amino-acid sequence at N-terminus of protein
site of protein synthesis determined by amino-acid sequence at N-terminus of protein
29
New cards
Protein translocation into ER
signal sequence recognized by SRP
SRP binds to receptor
polypeptide moves into ER lumen through a protein-lined pore
movement can occur co-translationally or post-translationally
SRP binds to receptor
polypeptide moves into ER lumen through a protein-lined pore
movement can occur co-translationally or post-translationally
30
New cards
Steps of Protein translocation into ER
1) Binding of SRP to signal sequence; SRP RNA wraps around rRNA / proteins of ribosomes and pauses translation
2) Attachment of ribosome to ER membrane through the binding of SRP to the SRP receptor
3) Binding of SRP to protein translocator leads to release of SRP. Translation resumes, seal is tight between ribosome and translocator
4) Cleavage of sequence signal
5) Protein is released in ER lumen
2) Attachment of ribosome to ER membrane through the binding of SRP to the SRP receptor
3) Binding of SRP to protein translocator leads to release of SRP. Translation resumes, seal is tight between ribosome and translocator
4) Cleavage of sequence signal
5) Protein is released in ER lumen
31
New cards
Signal Recognition Particle (SRP)
Made of six proteins and one RNA (other example?)
Binds to signal sequences
Binds to signal sequences
32
New cards
1st Step of Protein Translocation into ER
Binding of SRP to signal sequence / translation stopped
33
New cards
2nd Step of Protein Translocation into ER
Attachment of ribosome to ER membrane through the binding of SRP to the SRP receptor
34
New cards
3rd Step of Protein Translocation into ER
Binding of SRP to protein translocator leads to release of SRP. Translation resumes, seal is tight between ribosome and translocator
35
New cards
4th Step of Protein Translocation into ER
Cleavage of sequence signal
36
New cards
5th Step of Protein Translocation into ER
Protein is released in ER lumen
37
New cards
Integral membrane proteins
proteins that are at least partially embedded in the plasma membrane;
Transmembrane proteins can have different topologies (orientations)
Transmembrane proteins can have different topologies (orientations)
38
New cards
Translocation of lumenal/soluble protein
Signal sequence is recognized twice (by SRP and by translocator)
Signal sequence is 6-12 hydrophobic amino acids
Translocator is gated in TWO directions, cytoplasmic AND sideways bilayer opening
Signal sequence is 6-12 hydrophobic amino acids
Translocator is gated in TWO directions, cytoplasmic AND sideways bilayer opening
39
New cards
Start and Stop
__________________transfer sequences direct transmembrane proteins topologies
40
New cards
ribosome
SRP and its receptor interact to attach the ________________ to the ER membrane in close proximity to the translocon
41
New cards
lumen
Soluble proteins are released in the _______________ of the ER after cleavage of signal sequence by a peptidase.
42
New cards
start and stop
internal ________________ signal sequence are used for insertion of integral membrane proteins
43
New cards
integral membrane proteins
insertion of ____________________________ depends on the lateral gating of translocon
44
New cards
topologies
integral membrane proteins can have different _________________
45
New cards
Protein modification in the ER
- Lipidation
- Hydroxylation of amino acids (collagen)
- Disulfide bond formation
- Glycosylation
- Hydroxylation of amino acids (collagen)
- Disulfide bond formation
- Glycosylation
46
New cards
Lipidation
addition of lipid to a protein
Example:
- GPI-anchoring
Example:
- GPI-anchoring
47
New cards
Hydroxylation of amino acids
addition of hydroxy group (-OH)
(e.g., collagen)
(e.g., collagen)
48
New cards
Disulfide bond formation
intramolecular or intermolecular covalent bond between sulfur groups in cysteine aminoacids
Oxidation reaction
Happens in the ER lumen but not in cytosol:
- oxidizing environment
- protein disulfide isomerase
Oxidation reaction
Happens in the ER lumen but not in cytosol:
- oxidizing environment
- protein disulfide isomerase
49
New cards
Glycosylation
addition of carbohydrate (sugar) to a protein
50
New cards
N-linked glycosylation
Occurs in ER and Golgi
Starts in the ER:- a 14 sugar oligosaccharide is covalently attached
- transferred to Asparagine side chain (amide), therefore called _________________
Continues in Golgi
Starts in the ER:- a 14 sugar oligosaccharide is covalently attached
- transferred to Asparagine side chain (amide), therefore called _________________
Continues in Golgi
51
New cards
N-linked glycosylation in the ER
Addition of glycans to a lipid carrier
- 2 N-acetylglucosamine
- 9 mannose
- 3 glucose
Transfer to protein
co-translational, within (N-X-S/T) sequence
sugar transferred from lipid carrier
- 2 N-acetylglucosamine
- 9 mannose
- 3 glucose
Transfer to protein
co-translational, within (N-X-S/T) sequence
sugar transferred from lipid carrier
52
New cards
Oligosaccharyl transferase
enzyme that mediates transfer of assembled glycan
integral membrane protein with active site on lumenal side
one copy of enzyme per ER translocator
integral membrane protein with active site on lumenal side
one copy of enzyme per ER translocator
53
New cards
protein folding
unstructured chain of amino acid (inactive) to 3D folded structure (active)
54
New cards
what guides folding?
- all the information needed for the folding is contained in the amino acid sequence
- amino acids have different properties (charge, hydrophobicity)
- amino acids pack in such a way that the free energy of the protein arrives at a minimum
- amino acids have different properties (charge, hydrophobicity)
- amino acids pack in such a way that the free energy of the protein arrives at a minimum
55
New cards
Protein quality control: protein folding
- glycosylation state
- chaperone proteins
- ER lumen environment
- chaperone proteins
- ER lumen environment
56
New cards
Protein quality control
Main components:
1) calnexin, a chaperone
2) glucosidase, enzyme that removes glucose
3) glucosyl transferase, conformation sensing enzyme, will transfer a glucose back if protein is misfolded
4) mannosidase, removes mannose.
1) calnexin, a chaperone
2) glucosidase, enzyme that removes glucose
3) glucosyl transferase, conformation sensing enzyme, will transfer a glucose back if protein is misfolded
4) mannosidase, removes mannose.
57
New cards
Protein folding
_________________is reflected in glycosylation state
58
New cards
protein quality control
1) two glucose removed
2) remaining glucose binds to calnexin (chaperone), retains proteins in ER, give time to fold
3) glucosidase removes glucose, release protein
4) if protein is folded, it can leave ER. if not, conformation-sensing enzyme (glucosyl transferase) binds to misfolded protein and adds a glucose back.
2) remaining glucose binds to calnexin (chaperone), retains proteins in ER, give time to fold
3) glucosidase removes glucose, release protein
4) if protein is folded, it can leave ER. if not, conformation-sensing enzyme (glucosyl transferase) binds to misfolded protein and adds a glucose back.
59
New cards
cytoplasm
proteins that don't fold correctly are exported back into _________________ and degraded
60
New cards
degraded
proteins that don't fold correctly are exported back into cytoplasm and __________________
61
New cards
nonfolding protein
a slow-acting mannosidase enzyme that trims a mannose off the oligosaccharide tells the cell how long a protein has been in the ER
mannose-clipped protein can no longer be recycled and instead is sentenced to degradation
protein is exported from ER to cytoplasm
presence of N-glycosylation indicates to the cytoplasmic degradation machinery that the protein should be degraded.
mannose-clipped protein can no longer be recycled and instead is sentenced to degradation
protein is exported from ER to cytoplasm
presence of N-glycosylation indicates to the cytoplasmic degradation machinery that the protein should be degraded.
62
New cards
mannosidase
glucosyl transferase is fastest enzyme, then export machinery, and finally _______________ is the slowest
the relative rates of the enzymes key for the system to work.
the relative rates of the enzymes key for the system to work.
63
New cards
Unfolded protein response (UPR)
Under certain conditions, cells accumulates high levels of unfolded proteins in the ER
emergency action plan = _____________________
- Decrease translation
- Increase ER
- Increase number of chaperones
If ___________ is not sufficient, cell will trigger apoptosis
emergency action plan = _____________________
- Decrease translation
- Increase ER
- Increase number of chaperones
If ___________ is not sufficient, cell will trigger apoptosis
64
New cards
N-glycosylation
____________________ in the ER consists in the transfer of a 14 residues glycan
65
New cards
ER
Protein modifications occur at ______________
66
New cards
lumenal
protein folding in the ER depends on ______________ environment, chaperones, glycosylation state
67
New cards
chaperones
protein folding in the ER depends on lumenal environment, _____________ , glycosylation state
68
New cards
glycosylation
protein folding in the ER depends on lumenal environment, chaperones, _______________ state
69
New cards
N-glycosylation
_____________________ plays an important role in the protein quality control step (involving 4 main players)
70
New cards
Unfolded protein response (UPR)
Cells have an emergency action plan to deal with high level of unfolded protein, _________________.
71
New cards
Membrane trafficking
Most trafficking steps require a vesicle
72
New cards
Vesicle
A membrane bound sac that contains materials involved in transport of the cell.
73
New cards
Cargo protein
protein transported by a vesicle
74
New cards
ER to Golgi transport
Combination of:
- retention system
- retrieval system
- retention system
- retrieval system
75
New cards
Retention system
mostly based on physical properties of proteins prevent them from entering transport vesicles (e.g., too large)
Some proteins escape => retrieval system as backup
Some proteins escape => retrieval system as backup
76
New cards
retrieval system
The ER __________________
ER proteins therefore have a sorting signal (or retrieval signal) that will signal they should be kept and/or returned to ER
ER resident soluble proteins have a retrieval signal (KDEL)
- KDEL Receptor (integral membrane protein)
ER proteins therefore have a sorting signal (or retrieval signal) that will signal they should be kept and/or returned to ER
ER resident soluble proteins have a retrieval signal (KDEL)
- KDEL Receptor (integral membrane protein)
77
New cards
KDEL
_______________ receptor must function differently between ER and Golgi
- must bind ___________ proteins for retrieval in Golgi
- must not bind __________- proteins in ER, and must release proteins when it returns them to ER
How does this occur?
- the ER has a neutral pH, while the Golgi has proton pumps that makes it a more acidic environment
- the__________ receptor is very sensitive to pH
- is only active in acidic environment, so is active in Golgi
- is not active in neutral environment, so becomes inactive in ER, releasing proteins and not re-binding them
- must bind ___________ proteins for retrieval in Golgi
- must not bind __________- proteins in ER, and must release proteins when it returns them to ER
How does this occur?
- the ER has a neutral pH, while the Golgi has proton pumps that makes it a more acidic environment
- the__________ receptor is very sensitive to pH
- is only active in acidic environment, so is active in Golgi
- is not active in neutral environment, so becomes inactive in ER, releasing proteins and not re-binding them
78
New cards
Golgi complex
Stacked structure, flattened, disklike cisternae
possesses a -cis and -trans face
- cis face (incoming vesicles from ER, outgoing vesicles to ER)
- trans face (outgoing to many destinations in cell, incoming from endosomes)
possesses a -cis and -trans face
- cis face (incoming vesicles from ER, outgoing vesicles to ER)
- trans face (outgoing to many destinations in cell, incoming from endosomes)
79
New cards
Golgi Complex function
functions
- protein post-translational modification
- protein sorting
- protein post-translational modification
- protein sorting
80
New cards
Protein modification in golgi
Protein modifications
- phosphorylation (add phosphate)- glycosylation (N- and O-linked)
- sulfation (add sulfate)
- phosphorylation (add phosphate)- glycosylation (N- and O-linked)
- sulfation (add sulfate)
81
New cards
Why glycosylate?
Not protein quality control in this case
1) Can target proteins to specific compartments (lysosome)
2) Glycosylation introduces special structural qualities to proteins
- sugars are some of the most rigid macromolecules
- keep protein from being attacked by protease
- keep bacteria from approaching cell surface
1) Can target proteins to specific compartments (lysosome)
2) Glycosylation introduces special structural qualities to proteins
- sugars are some of the most rigid macromolecules
- keep protein from being attacked by protease
- keep bacteria from approaching cell surface
82
New cards
Trans Golgi Network
Sorting station for
- late endosome/lysosome
- early endosome/recycling endosome
- plasma membrane
- late endosome/lysosome
- early endosome/recycling endosome
- plasma membrane
83
New cards
ER to Golgi
Non ER resident proteins move from ______________________
84
New cards
retention and retrieval
o maintain unique compartmental composition, combination of ______________________ system.
85
New cards
vesicular transport
During _________________, cargo receptors bind to soluble cargo proteins
86
New cards
cis-Golgi
Proteins move from ______________ to trans-Golgi - two models
87
New cards
trans-Golgi
Proteins move from cis-Golgi to ___________- two models
88
New cards
Proteins
____________ get further modified in Golgi
89
New cards
Trans-Golgi network
______________ acts a a sorting station
90
New cards
Steps in Vesicle Transport
1) Vesicle formation
a) cargo recruitment
b) budding
2) Vesicle scission
3) Transport and targeting
4) Tethering
5) Fusion
a) cargo recruitment
b) budding
2) Vesicle scission
3) Transport and targeting
4) Tethering
5) Fusion
91
New cards
Vesicle formation
- coat and adaptor proteins
Adaptor proteins bind cargo receptors / integral membrane proteins
Adaptor proteins also recruit coat proteins
Coat proteins help deform the membrane
Adaptor proteins bind cargo receptors / integral membrane proteins
Adaptor proteins also recruit coat proteins
Coat proteins help deform the membrane
92
New cards
3 major type of vesicles
- clathrin-coated vesicles
- COPI-coated vesicles
- COPII- coated vesicles
- COPI-coated vesicles
- COPII- coated vesicles
93
New cards
Clathrin
vesicles dedicated to trafficking from Golgi complex, plasma membrane, and some endosomal compartments
94
New cards
COPI-coated
vesicles dedicated to trafficking at the Golgi complex
95
New cards
COPII-coated
vesicles dedicated to trafficking from the ER
96
New cards
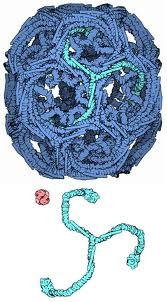
Clathrin
Stable basic unit is a triskelion
Triskelia then function as the basic unit for higher order ___________ assembly (cage or lattice)
Coat property - reversible self-assembly
Triskelia then function as the basic unit for higher order ___________ assembly (cage or lattice)
Coat property - reversible self-assembly
97
New cards
Vesicle scission
- Clathrin basket assembly cannot sever the lipid bilayer
- Dynamin is recruited to bud neck
- Dynamin cuts bud necks to free vesicle
Quickly after formation, vesicles loose their "coat"
This is the uncoating step
- Dynamin is recruited to bud neck
- Dynamin cuts bud necks to free vesicle
Quickly after formation, vesicles loose their "coat"
This is the uncoating step
98
New cards
Transport and targeting
Rab proteins act as address labels for vesicles
- more than 60 known Rabs, different Rab proteins target different organelles
- Rab proteins bind:
- Motor proteins, mediate movement in cell
-Tethering proteins, act to localize vesicle near target compartment
- more than 60 known Rabs, different Rab proteins target different organelles
- Rab proteins bind:
- Motor proteins, mediate movement in cell
-Tethering proteins, act to localize vesicle near target compartment
99
New cards
Rab proteins
___________ act as address labels for vesicles
Different __________________ guide movement to different organelles
Different __________________ guide movement to different organelles
100
New cards
Vesicle tethering
Tethering proteins are bound to target compartment
- recognize incoming vesicle
- assemble into long, multi-protein complexes that reach into cytoplasm
- bind to Rab and localize vesicle near target membrane
- recognize incoming vesicle
- assemble into long, multi-protein complexes that reach into cytoplasm
- bind to Rab and localize vesicle near target membrane