Covalent Bonds and Water's Biological Importance
1/63
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
64 Terms
Single Covalent Bond
Two electrons shared between two atoms.
Double Covalent Bond
More than two electrons shared between two atoms.
Triple Covalent Bond
Even more electrons shared between two atoms.
Molecule
Defined as two or more atoms held together by Covalent Bonds.
Compound
Requires two or more different elements in a defined ratio.
Electronegativity
A measure of how strongly an atomic nucleus attracts and holds onto electrons.
Nonpolar Covalent Bonds
Formed when electrons are shared equally between two atoms of the same type.
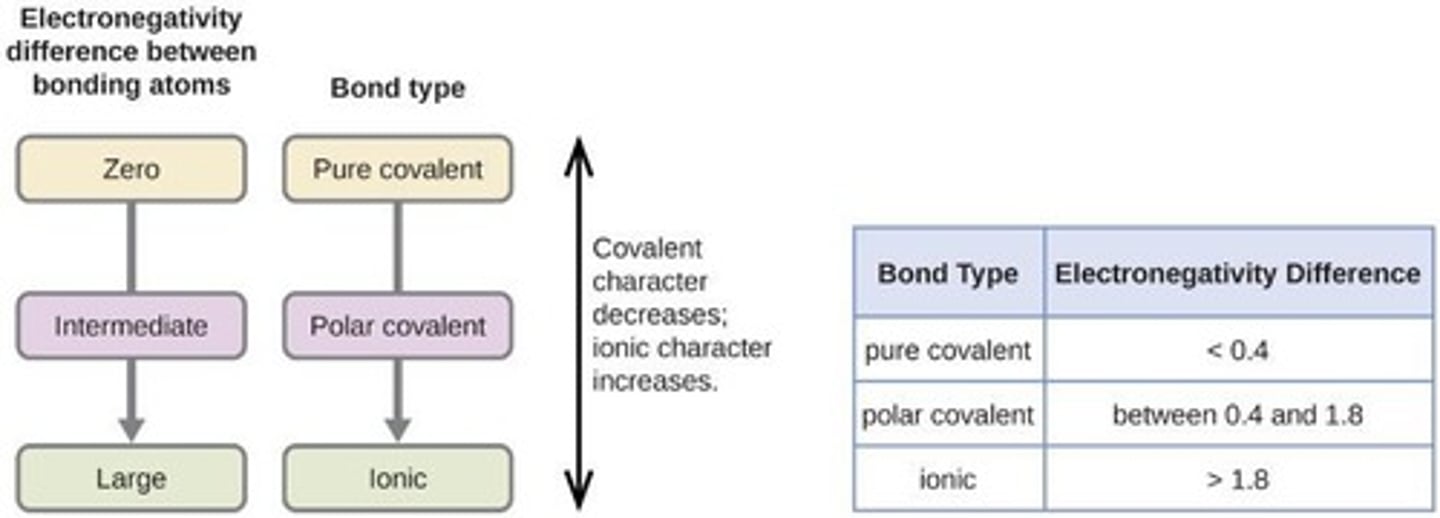
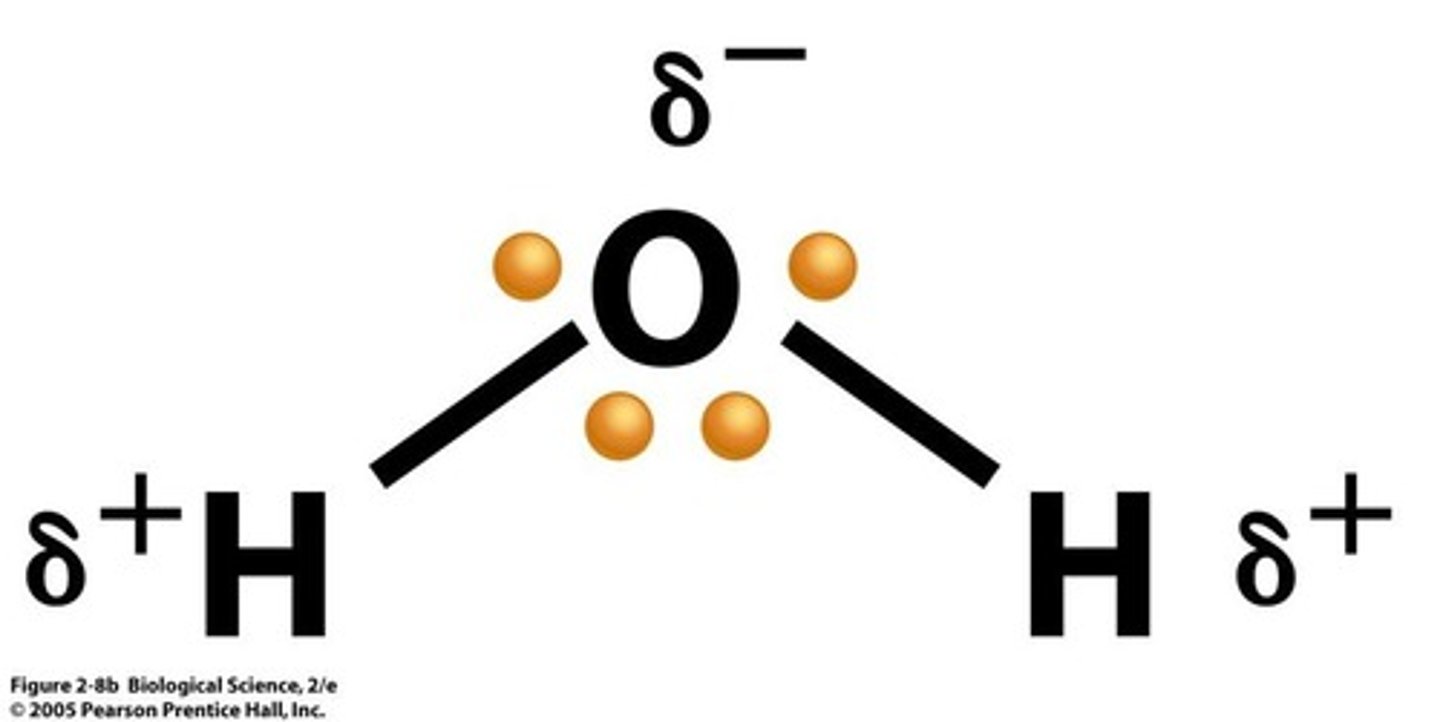
Polar Covalent Bonds
Formed when electrons are shared unequally between two different atoms.
Weak Bonds
Result from electrostatic attractions between two atoms.
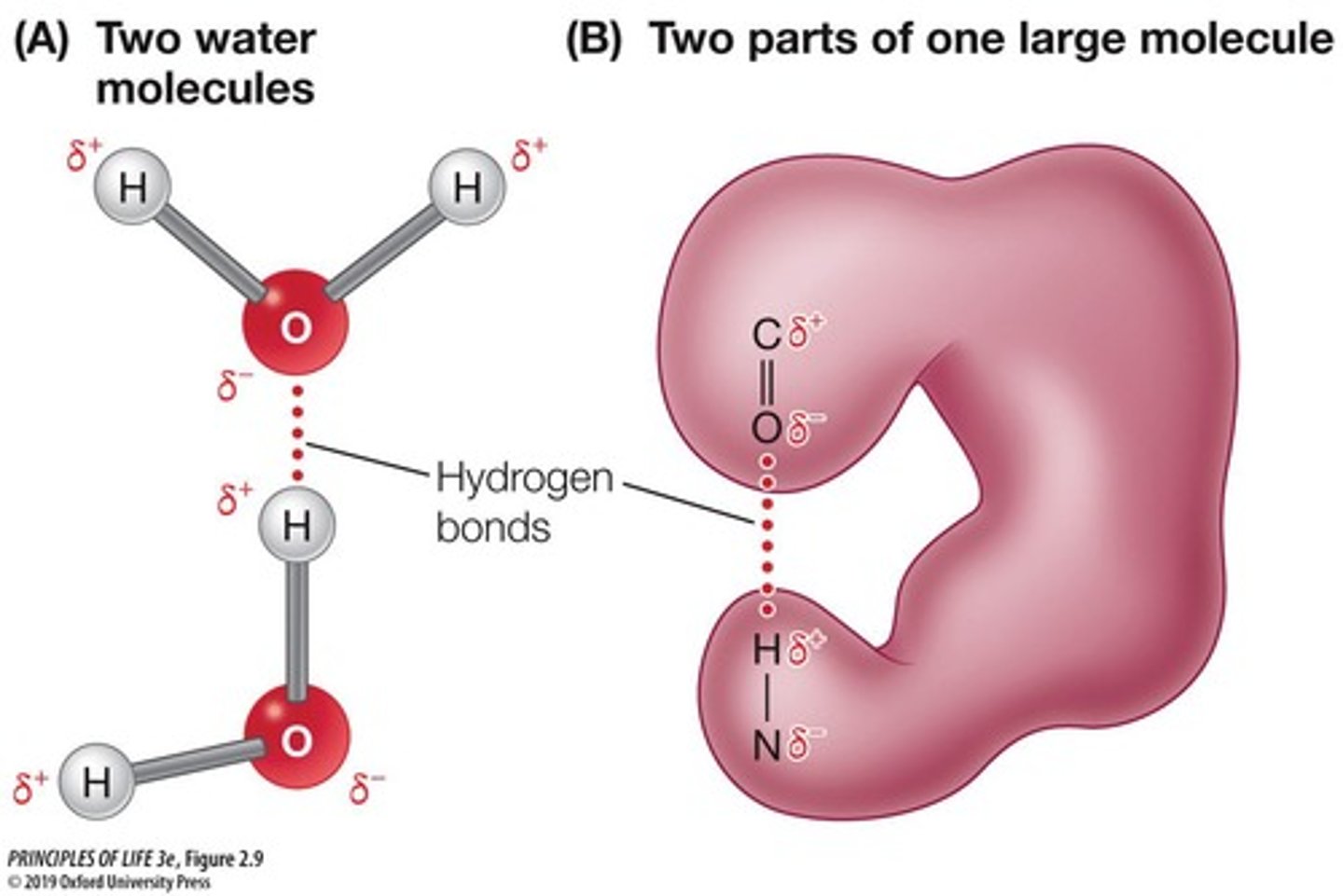
Hydrogen Bonds
Form when a H covalently bonded to one electronegative atom is attracted to another nearby electronegative atom.
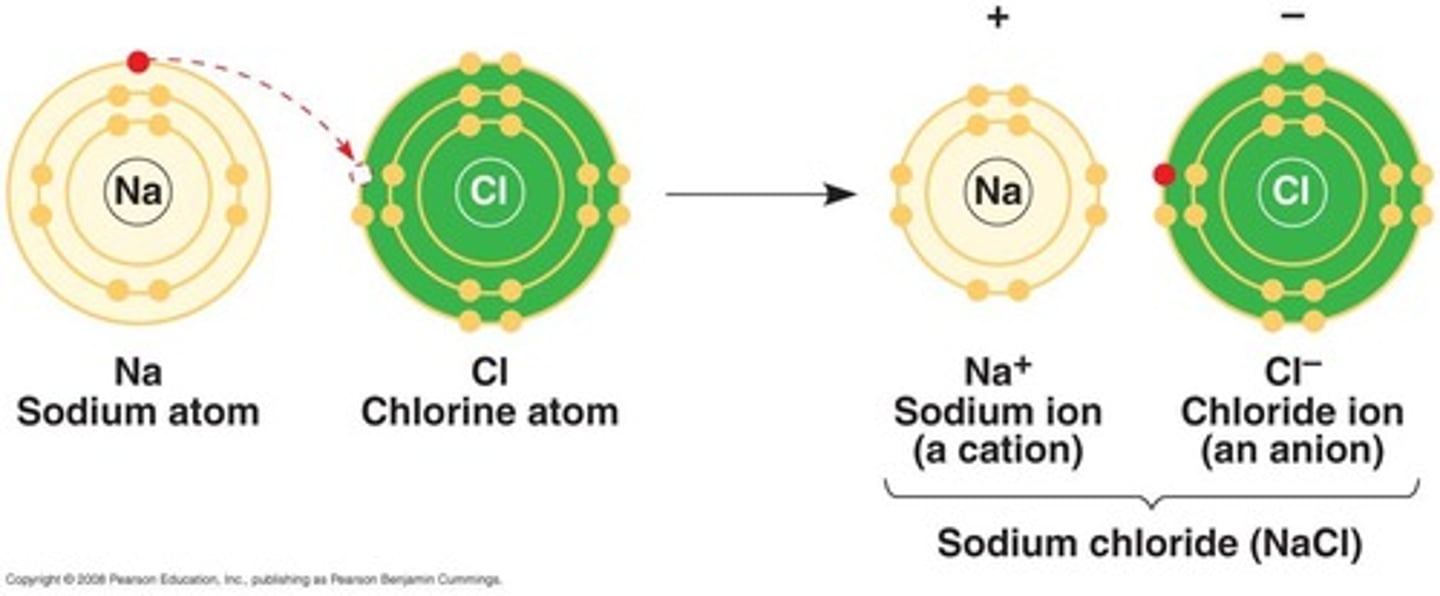
Ionic Bonds
Formed when the electronegativity difference is so large that it pulls electrons from the outer shell.
Salts
Compounds formed by Ionic Bonds.
NaCl
A compound that is not a molecule, formed by ionic bonds.
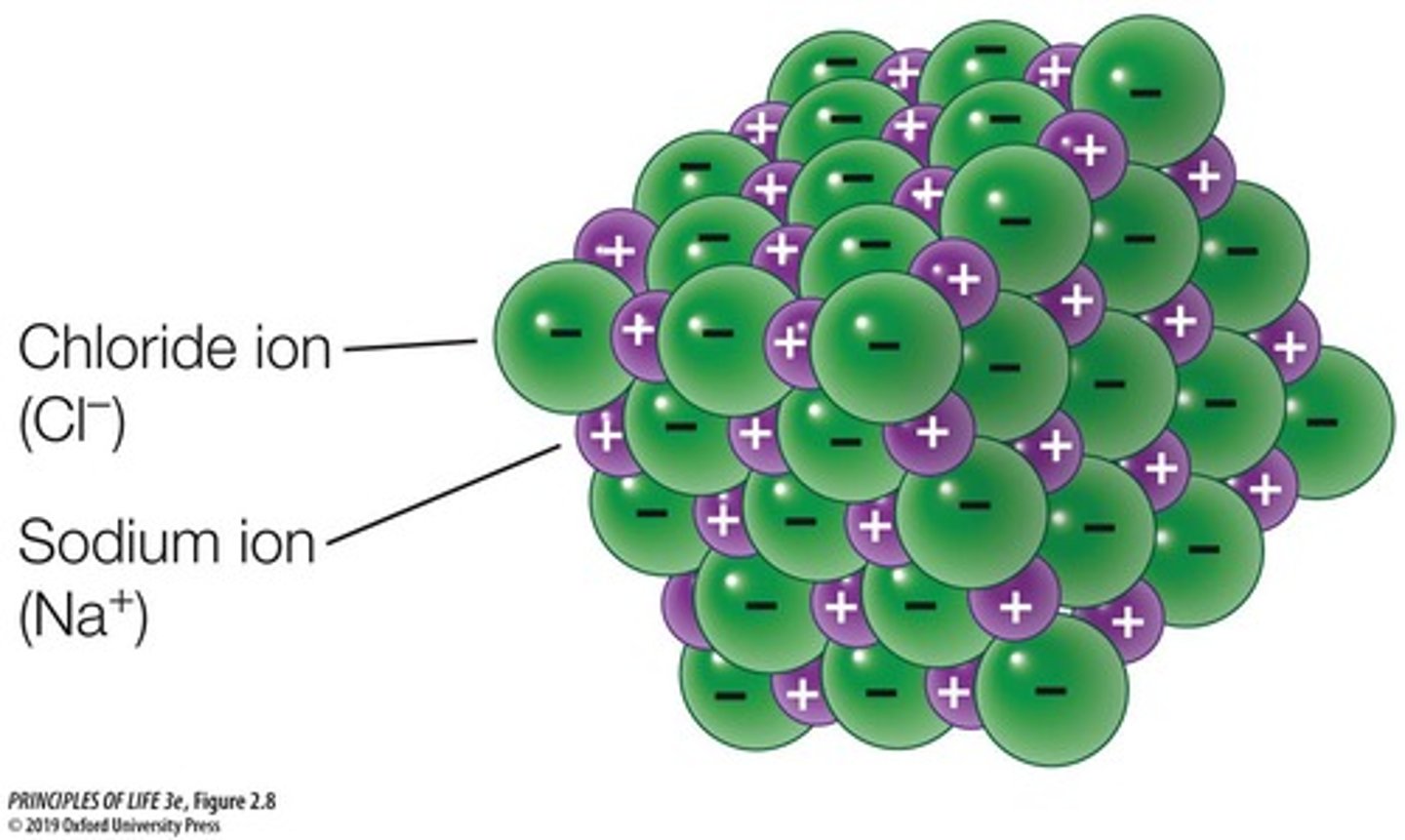
Ionic bond strength
Depends on conditions; strong in dry conditions and weak in wet conditions.
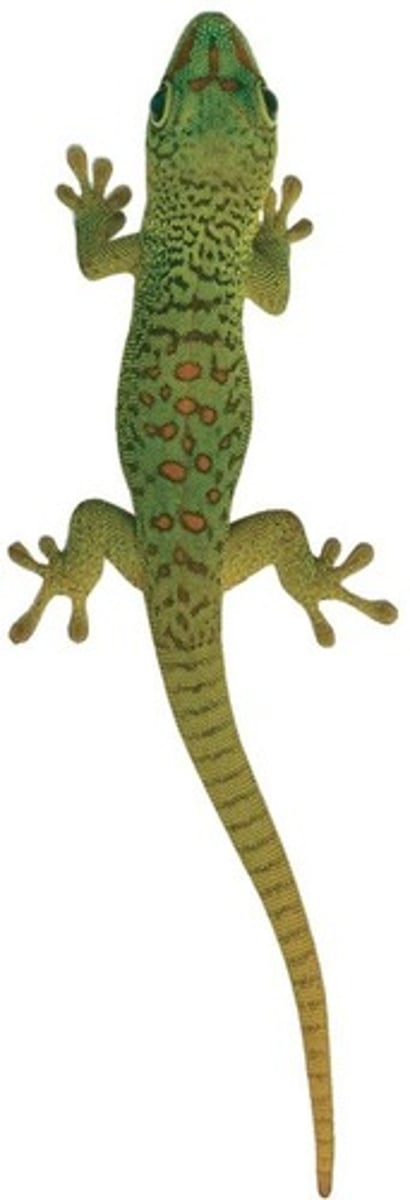
van der Waals Interactions
Weak interactions that occur between molecules with nonpolar bonds at extremely close range.
Setae
Microscopic 'hairs' on gecko toes that bind to surfaces via van der Waals interactions.
Water
Essential for life; organisms are composed mostly of water (65-75%).
Aqueous Chemistry
Refers to the chemistry of living organisms that is primarily based in water.
Aqueous Chemistry
Chemistry that involves water as a solvent.
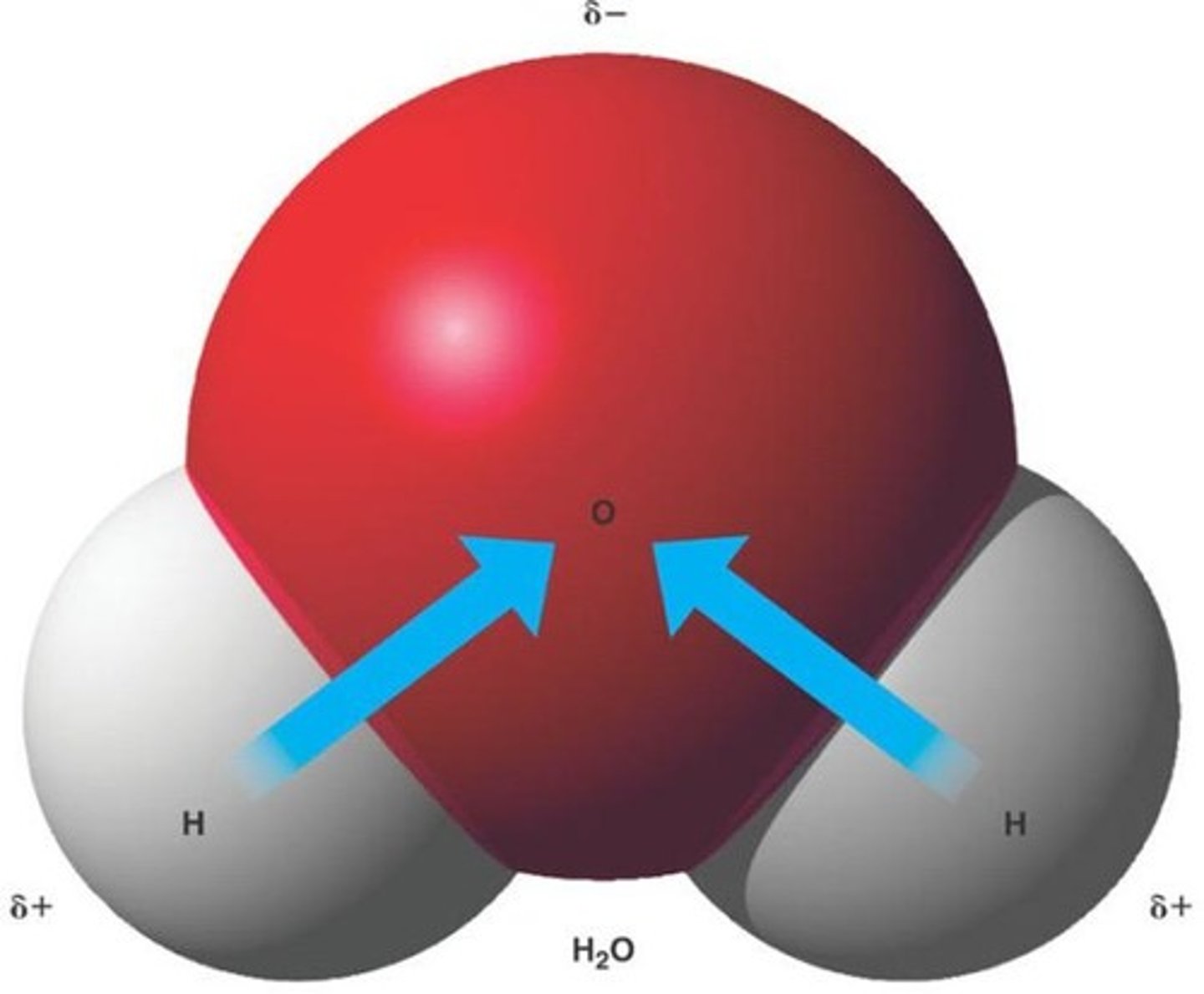
Polarity
The property of a molecule having partial positive and negative charges due to unequal sharing of electrons.
Hydrogen Bonds
Weak attractions between a hydrogen atom and an electronegative atom, allowing each water molecule to bond with up to 4 other water molecules.
Adhesion
The tendency of water molecules to stick to other substances.
Cohesion
The tendency of water molecules to stick to each other.
Specific Heat
The amount of energy required to raise the temperature of a substance.
Heat of Vaporization
The energy required to convert water from liquid to vapor.
Evaporative Cooling
The process where the energy needed to convert water from liquid to vapor transfers heat from the body to the air.
Density of Water
Solid H2O (ice) is less dense than liquid H2O, allowing ice to float.
Hydrophilic
Substances that are attracted to water and can dissolve in it.
Hydration Shell
The layer of water molecules that surrounds and interacts with solute particles.
Dissociation of Water
The rare process where water molecules split into H3O+ and OH- ions.
Mole
The mass in grams of the combined atomic masses of elements in a molecule; for water, 1 mole is 18 grams.
Concentration of Water
Pure [H2O] = 55.5 M (moles/liter).
Acid
A substance that increases the relative concentration of H+ in a solution.
Base
A substance that decreases the relative concentration of H+ in a solution.
pH Scale
A scale that expresses the acidity or alkalinity of a solution, ranging from 0 to 14.
Neutral Solution
A solution with a pH of 7.0.
Acidic Solution
A solution with a pH less than 7.0.
Basic Solution
A solution with a pH greater than 7.0.
Logarithmic Scale
A scale where each point represents a tenfold difference in concentration.
Sensitivity to pH Changes
Cells are sensitive to changes in [H+] because it can alter the ionization state of macromolecules, affecting their charge, structure, and function.
Carbon
Organisms on Earth are composed of molecules based largely on carbon.
Organic Chemistry
Carbon-Based Chemistry.
The Element Carbon
Elements are defined by the number of protons in the nucleus.
Carbon Protons and Electrons
Carbon has 6 protons (and 6 electrons).
Carbon Neutrons
Most common form has 6 neutrons.
Carbon Valence Electrons
4 electrons in outer (valence) shell.
Stability of Carbon
Carbon becomes very stable in a molecule by sharing 4 electrons.
Structure of Carbon
In a molecule, carbon is the center of an intersection with 4 branches.
Carbon-Based Molecules
Carbon can share one or more electrons with other carbons as well as with other elements.
Covalent Bonds
Carbon can form single, double, or triple covalent bonds.
Molecular Shape and Function
The type of bond determines molecular shape and function.
Hydrocarbons
Molecules consisting of ONLY Hydrogens and Carbons.
Properties of Hydrocarbons
All covalent bonds in a hydrocarbon are nonpolar.
Chemical Inertness of Hydrocarbons
Relatively chemically inert (Unreactive).
Solubility of Hydrocarbons
Relatively insoluble in aqueous solutions.
Isomers
Molecules with the same molecular formulae but with distinct arrangements of the atoms.
Structural Isomers
Differ in the covalent arrangement of atoms.
Geometric Isomers
Differ in the arrangement of groups across a C=C.
Enantiomers
Mirror-image isomer molecules.
Asymmetric Carbon
An asymmetric carbon forms covalent bonds with 4 different groups.
Pharmacological Importance of Enantiomers
Enantiomers can have different effects, as seen with drugs like Thalidomide.
Functional Groups
Small organic chemical groups that can substitute for one or more hydrogens in a hydrocarbon.
Effects of Functional Groups
Their presence increases the structural and functional diversity of the organic molecules.
Reactivity of Functional Groups
Make hydrocarbons more polar, soluble, and reactive.