Unit 8: Atomic Theory
1/61
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
62 Terms
Democritus’s theory
Matter is made up of tiny indivisible particles called atoms.
Aristotle’s theory
Everything is made up of 4 major elements: Earth, Air, Fire, and Water.
→ This theory was more popular because Democritus’s theory could not explain the various properties matter can possess. Also, it could not explain chemical reactions.
Billiard Ball Model of the atom
John Dalton reintroduced the idea of atoms and based it on experimentation.
He suggested each element has a specific type of atom with unique properties.
First “modern” atomic theory - John Dalton
Dalton’s atomic theory (1804)
All matter is composed of extremely small particles called atoms, which cannot be broken into smaller particles, created, or destroyed. → Proven WRONG!
The atoms of any given element are identical to each other and different from the atoms of other elements. → Proven WRONG!
Atoms of different elements combine in specific ratios to form compounds.
In a chemical reaction, atoms are separated, rearranged, and recombined to form new compounds.
Problems with Dalton’s atomic theory
1st statement: Works for chemical reactions but not nuclear reactions (where atoms can be destroyed).
2nd statement: There are different kinds of particles within a given element (Dalton didn’t know about isotopes, which are the same element but have different chemical properties).
→ Ex: Carbon has 3 forms.
First periodic table - D. Mendeleev (1869)
In 1869, Russian chemist Dmitri Mendeleev created the framework that became the modern periodic table, leaving gaps for elements that were yet to be discovered.
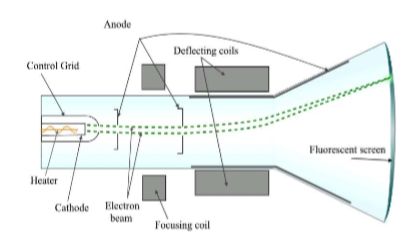
Electron discovery (1897), plum pudding model (1904) - Joseph John Thomson (J.J. Thomson)
He is credited with discovering the electron using aluminum plates in a cathode ray tube.
Atoms are not the smallest particles of matter.
Proposed the plum pudding model of the atom: Electrons are the negatively-charged plums in a homogenous, positively-charged pudding sphere.
Radioactivity was discovered and identified (1896 - 1899) - H. Becquerel, M. Curie, E. Rutherford
Marie Curie discovered radiation - the spontaneous splitting of atoms with unstable nuclei.
Ernest Rutherford discovered alpha radiation and that an alpha particle is a helium nucleus (He+ ion).
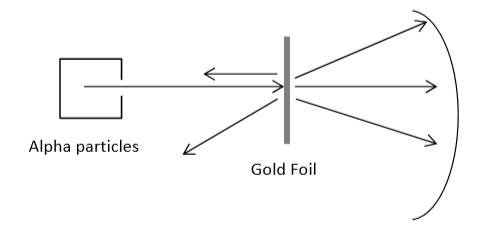
Proton existence was confirmed, and neutron was proposed (1919) - E. Rutherford
The nucleus has a positive charge from protons, which is equal to the number of electrons in a neutral atom.
Most of the mass of an atom is in the nucleus, the atom is mostly empty space (think a pin at the center of BC place).
Since the mass of an element does not equal the number of protons, there must be a neutral and dense particle (the neutron) in the nucleus. Chadwick formally discovered it.
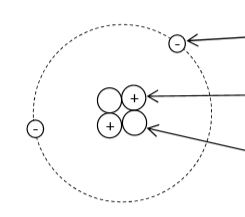
Atomic structure and isotope
Electron has a negative charge and almost no mass.
Proton has a positive charge and a mass of 1 atomic mass unit (AMU).
Neutron has a neutral charge and a mass of 1 atomic mass unit (AMU). Neutrons are responsible for the isotope of an atom.
Atomic number = # of protons in the nucleus. Each element has a unique atomic number.
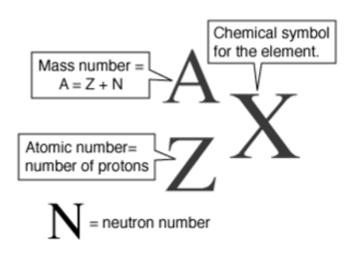
Isotopes
Different forms of the same element.
Have the same number of protons but different numbers of neutrons.
Written shorthand as: (Element)-(Mass #)
Mass number is always a WHOLE number.
In a specific isotope of an element, the Mass number = # protons + # neutrons.
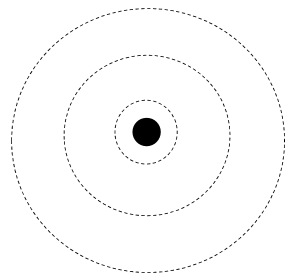
Bohr Model of Atom (1913) - Niels Bohr
Bohr took the Rutherford atom model and described the electron's movement around the nucleus.
Electrons orbit around the nucleus at set energy levels.
Each energy level corresponds to a “shell” or a quantum number, n.
The higher (further) the shell, the higher the energy.
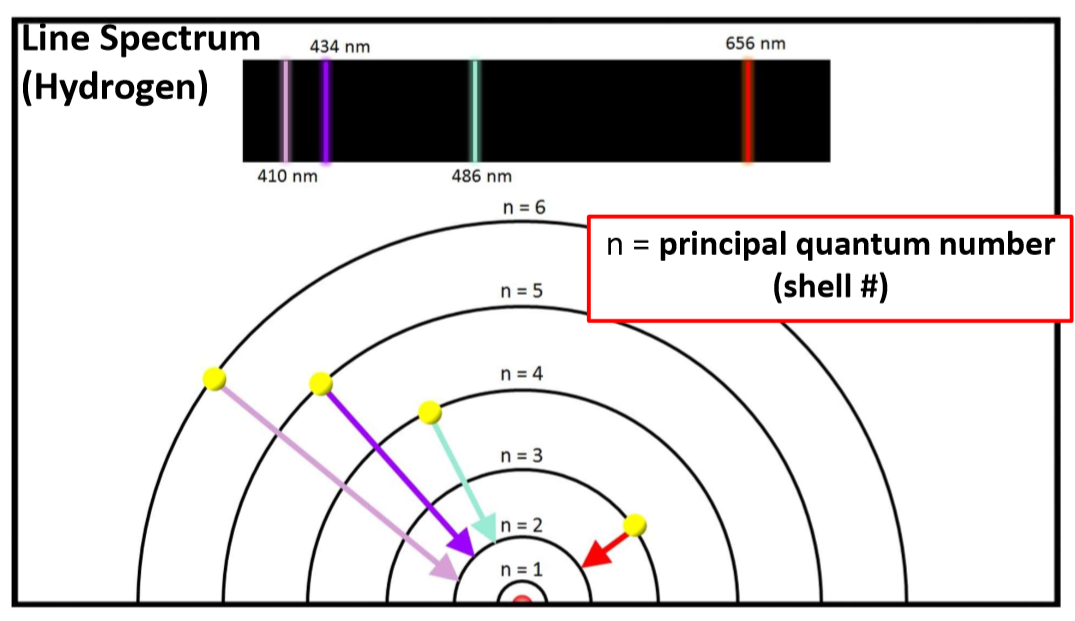
Emission Spectra
When a hydrogen atom is irradiated by energy, some of the energy is absorbed and light of a certain wavelength is emitted when it comes back down to its ground state.
If the light is passed through a prism, a “line spectrum” is observed.
Bohr’s interpretation
In 1913, Niels Bohr proposed a model that explained why the observed line spectrum for hydrogen looks the way it does:
The electron in hydrogen can only exist in specific energy states.
These energy states are associated with specific circular orbits that the electron can occupy around the atom.
When an electron absorbs energy, it instantaneously moves from one orbit to another.
The greater the energy, the farther the orbit is from the nucleus.
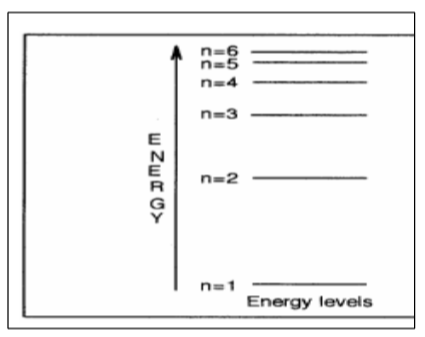
Energy levels
A specific amount of energy that an electron in an atom can possess.
The observed spectrum represents energy level differences occurring when an electron gives off energy and drops from a higher energy level.
Quantum energy: The energy difference between two different energy levels associated with the transition between the two levels.
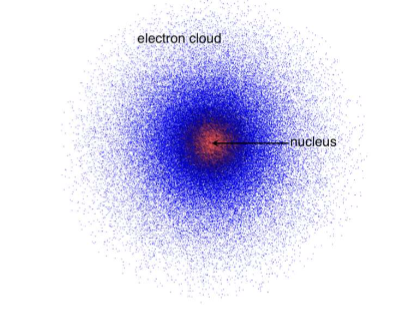
Erwin Schrodinger’s cloud model
Different electrons, depending on their energies, occupy particular regions of space called “orbitals”.
Orbital: The actual region of space occupied by an electron in a particular energy level.
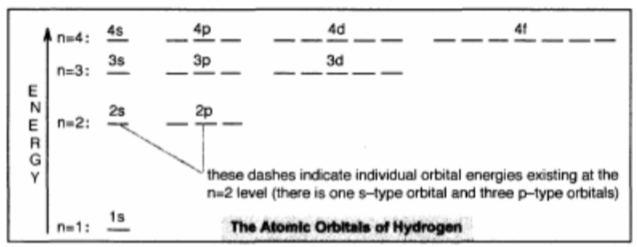
Energy Level Diagram for Hydrogen
Each dash represents the energy possessed by a particular orbital in the atom.
The letters s, p, d, and f refer to the four “types” of shapes or orbitals.
Shell: The set of all orbitals having the same n value.
→ Ex: The 3rd shell consists of the 3s, 3p, and 3d orbitals.
Subshell: A set of orbitals of the same type.
→ Ex: The set of five 3d orbitals in the 3rd shell is a subshell.
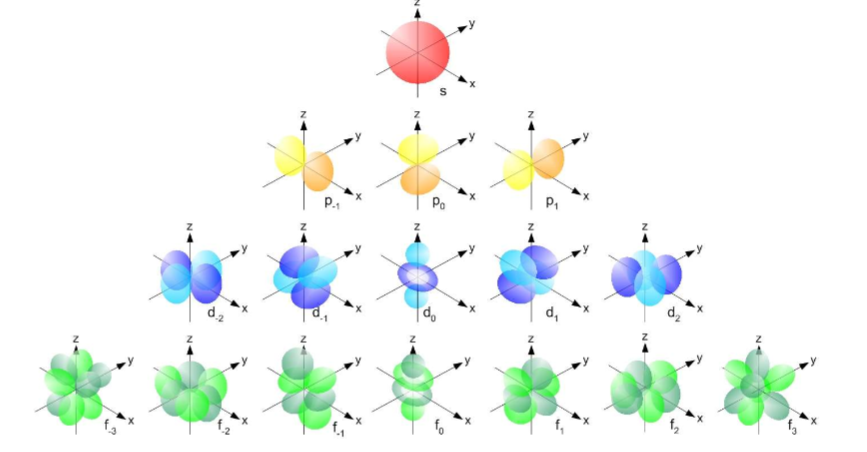
Rules governing which types of orbitals can occur
For a given value of “n”, n different types of orbitals are possible
n=1: only the s-type is possible
n=2: the s- and p-types are possible
n=3: the s-, p-, and d-types are possible
n=4: the s-, p-, d-, and f-types are possible
s-type subshell: 1 s-orbital (2 electrons)
p-type subshell: 3 p-orbitals (6 electrons)
d-type subshell: 5 d-orbitals (10 electrons)
f-type subshell: 7 f-orbitals (14 electrons)
Quantum numbers
1st quantum number (n): energy level or size of orbitals (1, 2, 3, …)
2nd quantum number (l): orbital shape (s, p, d, f)
3rd quantum number (ml): orbital orientation (x, y, z, axis)
4th quantum number (ms): spin of the electron (+1/2 or -1/2; “up” or “down”)
1st quantum number, n
n describes the shell or period number.
n can be 1 - 7.
n=1: the shell closest to the nucleus, lowest energy state for electrons.
n=7: the shell farthest away from the nucleus, the highest energy state for electrons.
2nd quantum number, l (lower case L)
l describes the shape.
l can be s, p, d, f (these are subshells).
Order of energy level is: s < p < d < f.

The Energy Level Diagram for Polyelectronic (having more than 1 electron) Atoms
The energy level of each subshell is not the same (like it is in Hydrogen) so the way we place electrons matters to the stability of the atom.
Rules for filling out orbital diagrams
Aufbau Principle: as atomic number increases, electrons are added to the available orbitals. To ensure the LOWEST POSSIBLE ENERGY for the atom, electrons are added to the orbitals having the lowest energy first.
Pauli Exclusion Principle: a maximum of TWO electrons can be placed in any orbital.
Hund’s Rule: when filling orbital subshells, each orbital must have a single electron in it first before doubling up with an electron of opposite spin.
Give electrons a spin with either an up or down arrow.
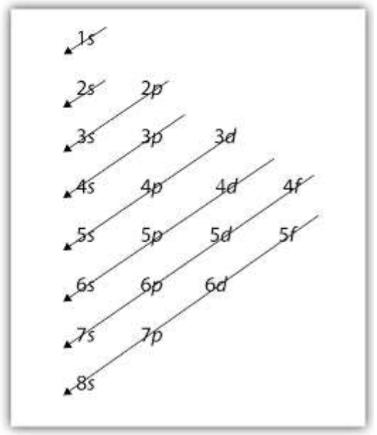
Writing Electron Configurations for Neutral Atoms
As atomic number increases, electrons are added to the orbitals having the lowest energy first … this is to ensure the LOWEST POSSIBLE ENERGY and most stable configuration for the atom.
A maximum of 2 electrons can be placed in each orbital (only one of each for each orbital).
Early Atomic Theory Timeline
~400 BC: The first coherent atomic theory - Democritus.
1804: First “modern” atomic theory - J. Dalton.
1869: First periodic table - D. Mendeleev.
1896 - 1899: Radioactivity discovered and identified - H. Becquerel, M. Curie, E. Rutherford.
1897: Electron discovered, plum pudding model (1904) - J.J. Thomson.
1909 - 1911: Identification of an atomic nucleus - E. Rutherford, H. Geiger.
1913: Mass of electron determined: R. A. Millikan.
1913: Positive charge in the nucleus and naturally occurring isotopes discovered - J.J. Thomson.
1913: Atomic number determined, periodic table reorganized - H. Moseley.
1913: Bohr Model of the Atom - N. Bohr.
1919: Proton existence confirmed, neutron proposed - E. Rutherford.
1931: Neutron identified - J. Chadwick
Periodic Table “Shortcut”
Column: tells how many electrons are in the orbital
Row: the principal quantum number (shell #)
CORE electrons
The set of electrons with the configuration of the nearest noble gas having an atomic number LESS than that of the atom being considered.
Negative Ions (anions)
Add electrons to the last unfilled subshell, starting where the neutral atom left off.
→ Ex: O2- - ([He] 2s2 2p4) + 2e → [He] 2s2 2p6
If you want to write the core notation for a noble gas such as Kr, you must show the electron configuration of the LAST full shell (i.e. the previous noble gas).
→ Ex: Ar - [Ne] 3s2 3p6
Positive Ions (cations)
Electrons in the outermost shells (largest n value) are removed first.
Removal order for the same n-value: p-electrons BEFORE s-electrons BEFORE d-electrons.
→ Ex: Fe [Ar] 4s2 3d6 → Fe2+ [Ar] 4s0 3d6
VALENCE / OUTER electrons
Electrons in the current or unclosed shell are involved in chemical reactions.
Found in the outermost shell.
Are all the electrons in the atom EXCEPT:
Core electrons
In filled d- or f- subshell
→ Ex: Al ([Ne] 3s2 3p1) has 3 valence electrons
Isoelectronic
Same e-configuration as another particle.
→ Ex: Na+, Ne, and O2- are isoelectronic because they are all 1s22s22p6
Electron Configuration Rule Exceptions
Cr ([Ar] 4s1 3d5) - “4s1” and “3d5” are two half-filled subshells.
Cu ([Ar] 4s1 3d10) - “4s1” is half-filled subshell, and “3d10” is filled subshell.
Period
The set of elements in a given row going across the table.
Group / Family
The set of elements in a given column going up and down the table.
Elements in the same chemical family have similar chemical properties because they have similar outer electron configurations.
Group 1 - Alkali Metals
Very reactive
Reactivity increases down the group
Tend to form 1+ ions
Group 2 - Alkaline Earth Metals
Less reative than alkali metals
Reactivity increases down the group
Tend to form 2+ ions
Group 3-12 - Transition Metals
d-orbitals filled last
Exception: Cu & Cr
Many possible ions (multivalent)
Group 13 - 16
Group 13 (Boron group): Tend to form 3+ ions (e.g. Al3+)
Group 14 (Carbon group): Tend to form 4+ ions (but C may also form 4- ions) (e.g. Si4+)
Group 15 (Nitrogen group): Tend to form 3- ions (e.g. N3-)
Group 16 (Oxygen group): Tend to form 2- ions (e.g. O2-)
Group 17 - Halogens
Very reactive
Reactivity increases going up the table
Tend to form 1- ions
Group 18 - Noble gases
Very low reactivity, very stable
Completely filled outer orbital
Other elements tend to form ions that are isoelectronic to the nearest noble gas
Hydrogen
Share properties with many different families
Metals
Solid at room temperature (Hg is liquid).
Shiny or lustrous when freshly cut or polished.
Good conductors of electricity or heat.
Generally malleable (can be hammered into thin sheets) and ductile (can be stretched into wires).
During chemical changes, metals tend to give up e- to form cations.
Non-Metals
Usually gases or brittle solids at room temperature (Br is liquid).
Poor heat and electricity conductors.
Solid non-metals are dull to lustrous in appearance and opaque to translucent.
During chemical changes, non-metals tend to gain e- from metals to form anions or share e- with other non-metals.
Metalloids
Share properties from both metals and non-metals.
As temperature increases:
Metals → conductivity decreases
Metalloids → conductivity increases
B, Si, Ge, As, Sb, and Te are confirmed as metalloids.
The following 2 elements are sometimes classified as metalloids:
Polonium (Po): metal OR metalloid
Astatine (At): non-metal OR metalloid
Ionic Bonds
Metal bonded to non-metal.
Forms between 2 atoms with large differences in electronegativity (EN) and ionization energy (IE).
The transfer of valence electron(s) from the atom with lower IE and EN to the one with greater IE and EN.
Electrostatic attraction occurs when the 2 atoms become ions after the e-transfer (e.g. NaCl).
Closer charged particles = Greater attraction.
Greater charge = Greater attraction
Can form structures known as crystal lattices. The vast number of attractive forces → High melting temperatures for ionic compounds.
Ionic bonds form when the difference in electronegativity (ΔEN) > 1.8 OR if it is between a metal and a non-metal.
Non-polar Covalent Bonds
Occurs between 2 identical non-metal elements → Their EN values are equal.
Also applies to different elements with the same EN.
Collision of these 2 atoms will cause the electron clouds to overlap.
If there is enough overlap, attractive forces (nucleus) may begin to overlap repulsive forces (electrons). The 2 atoms will settle into a position next to each other where a pair of valence e- sits between both nuclei.
The attraction experienced by both nuclei results in a covalent bond (e.g. H2).
ΔEN = 0 → The pair of electrons will spend their time equidistant between both nuclei (i.e. the electrons will not be more attracted to one nuclei or the other).
Bonds are considered non-polar if ΔEN < 0.5.
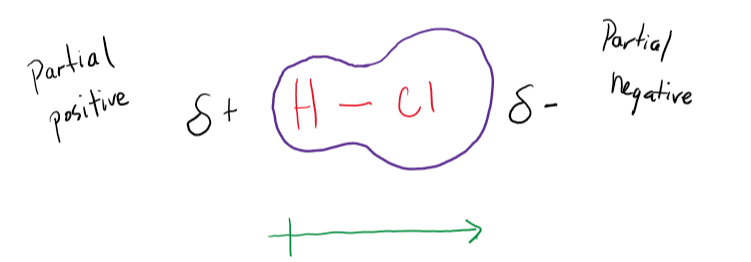
Polar Covalent Bonds
Formed when there is an unequal sharing of electrons.
Dipole: When electrons are pulled more towards one atom due to higher EN, one side of the molecule becomes more negative than the other side.
Covalent compounds tend to have lower melting points than ionic compounds.
Bonds are considered POLAR covalent if 0.5 < ΔEN < 1.8.
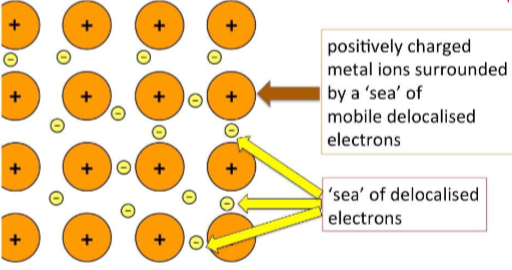
Metallic Bonds
Positively charged metal ions surrounded by a ‘sea’ of mobile delocalized electrons.
→ Allows metals to be malleable and good conductors.
Forms metallic crystals.
Octet rule
All atoms will form a stable octet (8 electrons around each atom) upon bonding because it provides the most stable, low-energy configuration for both atoms of a bond.
Ionic Compounds
Metals - the total number of dots representing # of e- that can be lost to form a complete octet.
Covalent Molecules
Non-metals - the number of unpaired electrons shows the amount that it must gain to form a complete octet.
Atoms that VIOLATE the Octet Rule
Hydrogen only makes one bond.
B, Be, and Al (will have less than a full octet).
3rd and 4th-period elements like P and S (may have an expanded octet).
Periodic Table Trends
Electrons in large orbits are farther from the nucleus and experience less attraction because of the shielding from the inner shells.
More protons = more attraction for electrons → effective nuclear charge (Zeff), how much “pull the e- feel from the nucleus”.
Electrons in the same subshell repel each other.
A trend is a general relationship that can have many exceptions!
Atomic radius (AR)
Measure the radius from the nucleus to the outer (valence) electrons in an atom.
Down a group → AR increases in size; more shells (higher n values) and more shielding.
Across a period → AR decreases because of # protons increase but added e- join the same orbital (so e- “feel” more attraction to the nucleus) and are held in tighter.
Ionic radius - AR of an ion
Ionic radii of cations (+) of an element are generally smaller than their neutral atoms (fewer e-).
Ionic radii of anions (-) are generally larger than their neutral atoms (more e-).
Atoms having the same e-configuration but different # protons will have different Zeff.
→ More protons = higher Zeff
Ionization energy
Minimum energy needed to remove an electron from an atom.
First ionization energy (IE1) is the energy required to remove the first electron from an atom. IE2 for a second e-, etc.
This property is directly related to the attraction of the electrons to the nucleus. The trend is the opposite of atomic radius (i.e. highest IE numbers belong to the smallest atoms and large atoms have low IE).
AR - Metals > AR - non-metals → Metals have lower IE. This is why they tend to LOSE e- (form cations) and non-metals tend to GAIN (form anions) or share e-.
Electronegativity (EN)
A measure of the attraction an atom has for a shared electron.
VERY important for chemical bonding.
A measurement of an atom Zeff on OTHER atoms.
Atoms with high EN tend to pull bonded electrons closer to their nuclei.
Noble gases have no EN value.
EN will have the same trend as ionization energy.
Metallic character
Down group = more metallic
Across period = less metallic
Intermolecular forces
Weaker than intramolecular forces.
The interaction between intermolecular forces describes how molecules interact with each other.
The strength or weakness of intermolecular forces determines the state of matter of a substance (e.g. solid, liquid, or gas) and some of its chemical properties (e.g. melting point, structure).
3 main intermolecular forces are: London dispersion force, dipole-dipole interaction, and hydrogen bonding.
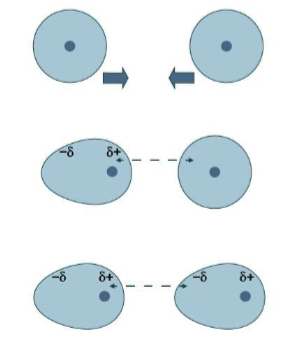
London dispersion force
The weakest of intermolecular forces.
A temporary force between two nonpolar molecules.
The electrons of one molecule are attracted to the nucleus of the other molecule, while repelled by the other molecule’s electrons.
A temporary dipole is induced when the attractive and repulsive electrostatic forces distort the electron clouds of the molecules.
→ Ex: The interaction between nitrogen gas (N2) and oxygen gas (O2) molecules.
London dispersion force (next)
Other names: London forces, dispersion forces, instantaneous dipole forces, induced dipole forces, or the induced dipole-induced dipole force.
The electrons of the atoms are not only attracted to their atomic nucleus but also to the protons in the nucleus of the other atoms.
London forces are always PRESENT and the only force of attraction between non-polar molecules.
Greater atomic number = Stronger London forces (because the temporary dipole will increase as # electrons increase).
→ Ex: London force in I2 > London force in Cl2
The total force due to London forces will be greater in larger (longer) molecules because there are more places for attraction to occur.
Covalent substances with only London forces holding units together have low melting and boiling points.
Hydrogen bonding
A specific example of a dipole-dipole interaction involving hydrogen.
Results from the attractive force between a hydrogen atom covalently bonded to a very electronegative atom such as N, O, or F atom and another very electronegative atom.
Responsible for:
Expansion of ice when water freezes
Surface tension of liquid water
Aquatic life
Protein surface
DNA structure
→ H- bonding means liquid water has as many intermolecular bonds and intramolecular ones.