what is the equiibrium constant Kc
1/14
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
15 Terms
What does Kc tells us ?
It tell us the position of equilibrium
Use essentially a ratio between the products and the reactants
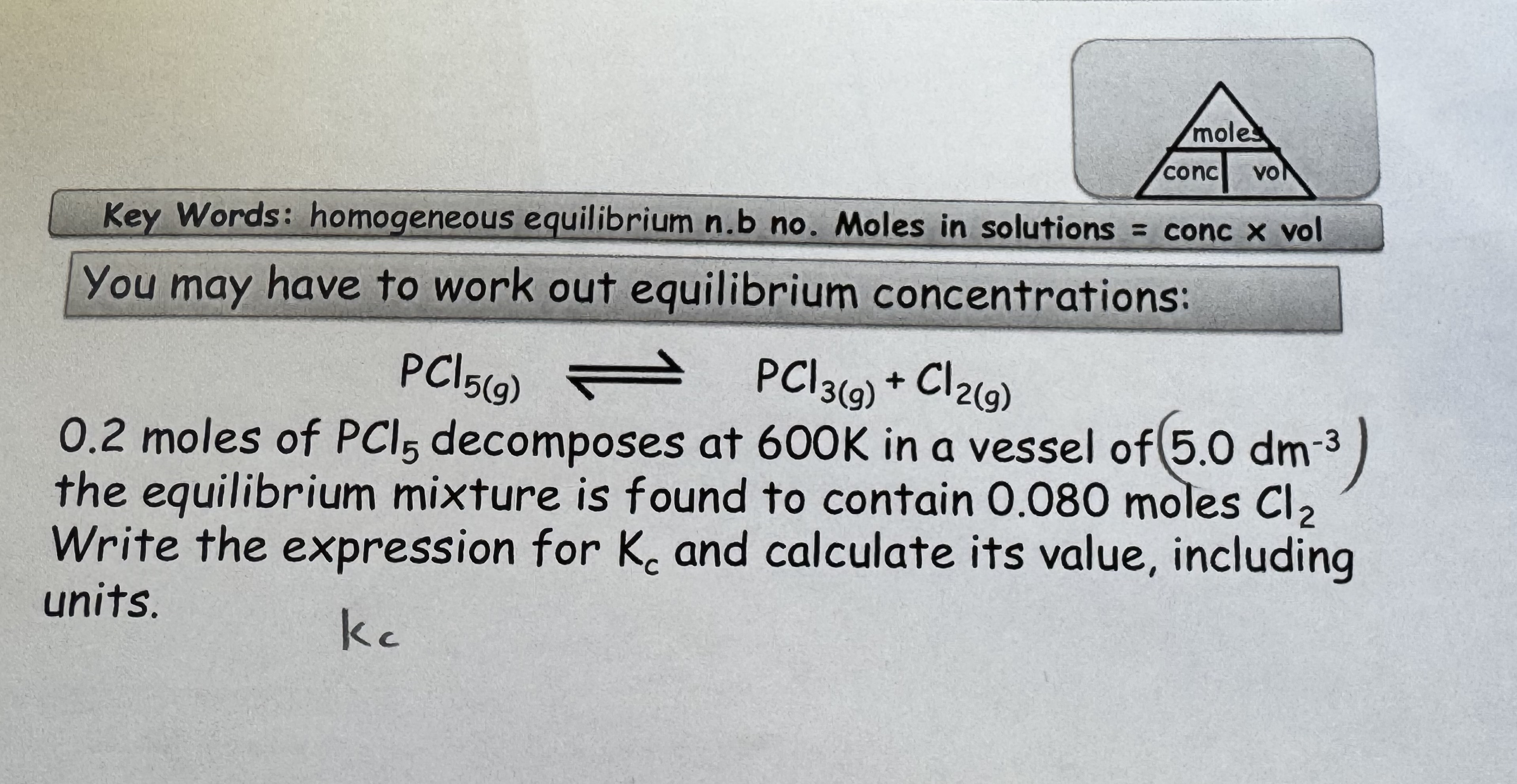
What is the equation for Kc ?
Kc = products / reactants
N2O4 ← → 2 NO2
Kc = [ NO2 ] 2 / [ N2O4 ]
Work out the units for Kc ? N2O4 ← → 2 NO2
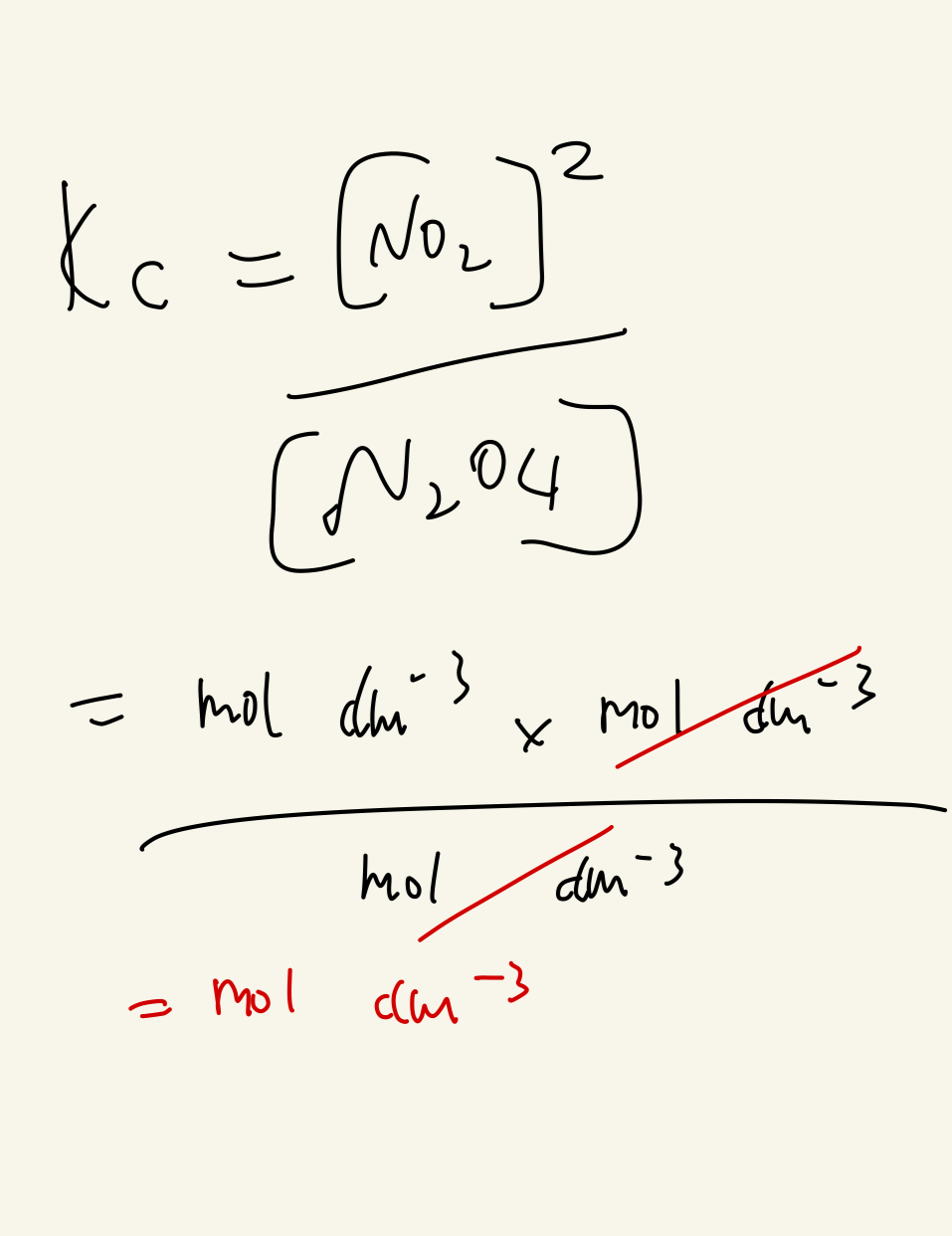

[ 0.320X 0.320 ] / [ 0.140 ] X [ 0.0400 ] = 18.3 ( no units )

Kc = [ 0.80 ] 2 / 0.10 X 0.10 = 64 no units
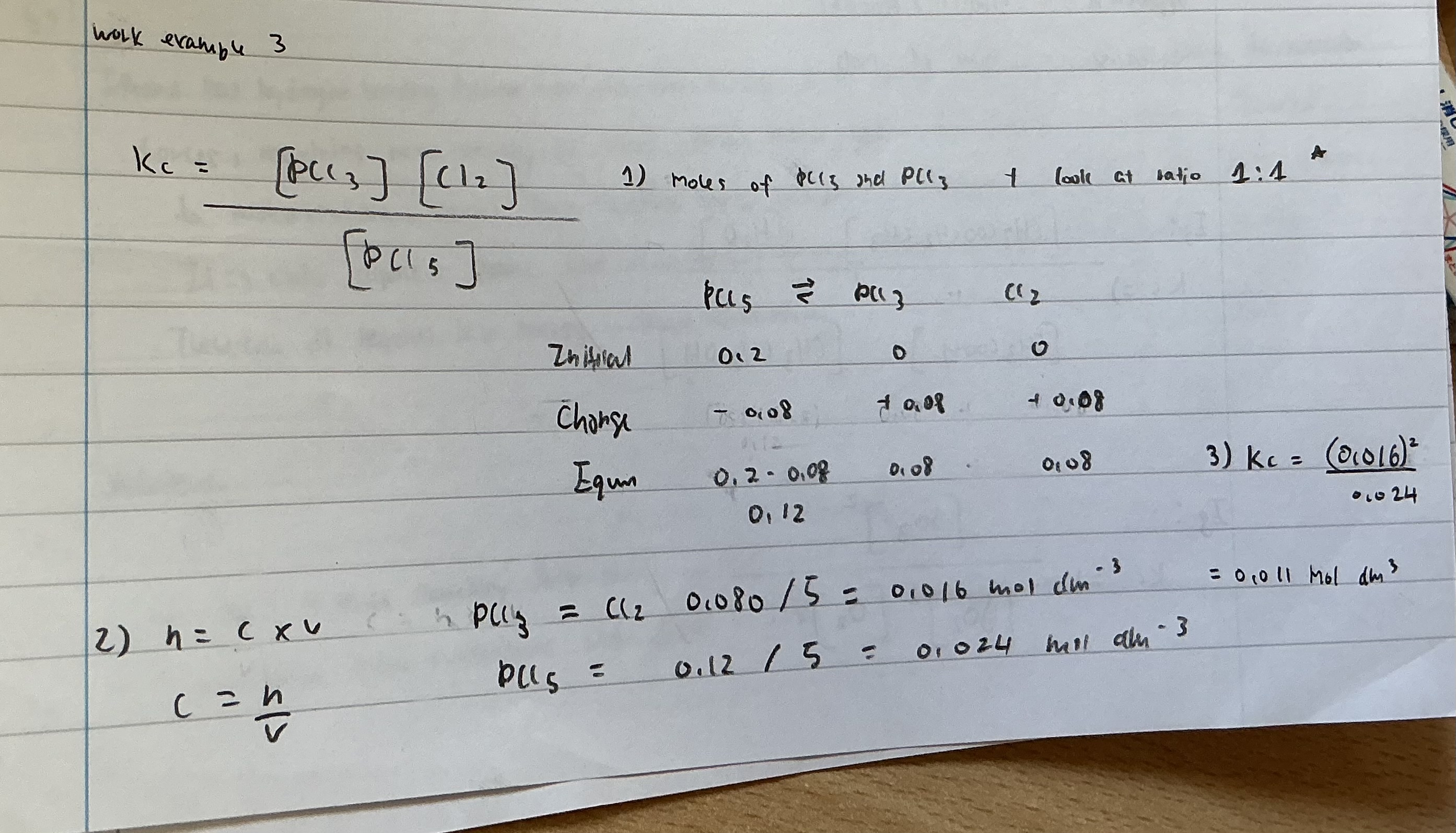
Two disturbance which would results in a decrease in the concentration of chorine are : Cl2 + H2O = HCIO + HCl
Increase in con.c / volume of water
Increase in products
Two disturbances which would results in an increase in the concentration.c of chorine are : 4 HCi + O2 = 2 Cl2 + 2 H2O ( exothermic reaction )
Increase in concentration of Cl2 and increase in concentration of either reactants, removal of water in system to cool down system
Two disturbances which could be made without changing the amount of reagents or products in the system which would results in a shift of the eqm to the right are : PCI5 = PCI3 + CI2 ( endothermic reaction )
Decreases in pressure
Increase in temperature
Two disturbances which would results in an increase in the % yield of ethanol are : CH=CH2 + H2O ← → CH3CH2OH
( exothermic reaction )
Increases in pressure of the system and eqm shifts to right by decreasing in temperature
Two disturbances which would results in no change in the position of the eqm are : HCOOH + CH3OH ← → HCOOCH3 + H2O ( 0 KJ mol -1 )
Addition of catalyst and change in system temperature as the enthalpy change is 0




