Schizophrenia
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
Neurological vs Neurodegenerative
When circuits in the brain that regulate function break down it leads to neurological disorders
Neurological disorders have other causes for the dysfunction, but the consequence is that the communication is disrupted
Schizophrenia is a neurological disease
Neurodegenerative is due to cell death
Scizophrenia
Characterised by hallucinations, paranoia
Can be precipitated by a certain event – could be that the connection is weak initially causing the person to appear asymptomatic, but a precipitating event can cause this connection to strengthen leading to onset of symptoms.
Role of stress in precipitating the illness in vulnerable people
Episodic in nature – periods of time the individual is unaffected, periods of time where the individual can be severely impacted.
Episodes may link to stress – implies theres a complex relationship etween the environmental cause and the genetic vulnerability. The genetic component sets a threshold and the environment tests this threshold.
Symptoms
Symptoms are separated into positive and negative symptoms, usually with the onset of positive symptoms first
Positive – not positive in that they're good but they do things that they wouldn’t normally do
Hallucinations (mainly auditory)
Thought disorders - E.g: might think that someone is trying to hurt you
Stereotyped behaviours – E.g: disorganised speech or disturbances in behaviour
Negative – things that individuals would normally do that they now do not do
Poverty of affect – individuals become antisocial, emotionless, flat, immobile
Cognitive impairment
Temporal disorientation
Positive symptoms may be due to the excess of neurotransmitter release whilst the negative symptoms may be due to lack of neurotransmitter release.
time course of symptoms
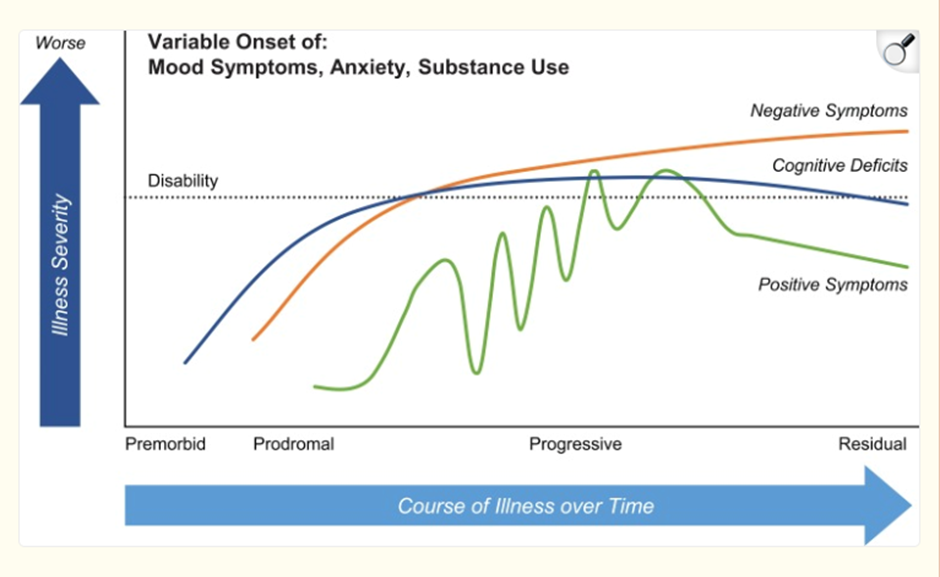
cognitive deficits accompany negative symptoms
Can be difficult to untangle these two as if you’re a clinician examining these patients can be hard to tell if the person doesn’t know the answer to your question or simply doesn’t want to answer
Incidence of Schizophrenia
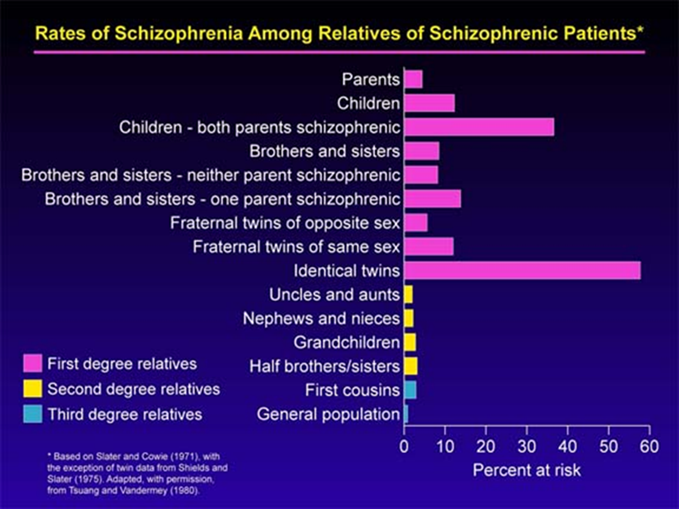
High incidence of schizophrenia in first degree relatives compared to second- and third-degree relatives – suggests that likely a strong genetic component
Not 100% chance of identical twins both being schizophrenic, suggests that there's also environmental contributors
Development theory of Schizophrenia
Suggests that the etiology of schizophrenia may involve pathological process (occuring as late as the second or third trimester) caused by environmental and genetic factors which before the brain approaches its adult anatomical state
Neurodevelopmental abnormalities have been suggested to lead to the activation of pathologic neural circuits durng adolescence or young childhood
The “2 hit model” suggests that maldevelopment during 2 critical timepoints:
early developmental insult —> dysfunction in specific neural networks
A later environmental trigger which causes the onset of full symptoms by disrupting brain signalling
Evidence for the Developmental Theory
Congential evidence
Presence of low-set ears, epicanthal eye folds, and wide spaces between the first and second toes are suggestive of first trimester anomalies
If this is true you would see evidence of this in the adult brain but the brain of schizophrenics not that different to brain of normal person
increased frequency of obstetric and perinatal complications in schizophrenics
Evidence in the adult brain
Eventually found architectural abnormalities in brain regions of schizophrenics – specifically in the entorhinal cortex
Decreased number in small neurons in the superficial layers of the cortex
Increased numbers of large neurons in deep layers
the superficial layers of the cortex are especially important for context processing and integration of information which is disrupted in schizophrenia
Difference in cortical thickness in early life – can be used as an indicator if an individual will become schizophrenic. Initially, the cortex is very thick but due to synaptic pruning it thins over time.
Causes of Schizophrenia which play into the developmental theory
Genetics
Pyschosocial
Structural Brain Damage
Viral infection
Genetic component
Proven by high concordance in monozygotic twins (48%)
Experiments done where schizophrenic mothers gave their children up for adoption to mothers without the condition resulted in a low incidence of schizophrenia. This shows that the environment in the home plays a part in precipitating this illness
Schizophrenia considered a pathway disease – one gene is not strong enough to influence the pathway; there are many pathways that contribute to behaviours which cause schizophrenia. Requires either multiple genes in one pathway to be affected or multiple genes in multiple pathways (more likely to be true).
Shows that there is a certain resilience in these pathways as it requires many genes in many pathways in order to influence the neurochemistry of behaviour.
Environment acts as the modulator of the risk. Can have inherited these multiple loci but is ultimately dependent on the environment.
Pathway associated studies (PWAS) look at the pathways associated with schizophrenia. AI can help to identify high/low risk pathways for schizophrenia.
The vast majority of schizophrenics have a high number of inherited risk loci and an environmental exposure. The opposite is true for non-schizophrenics.
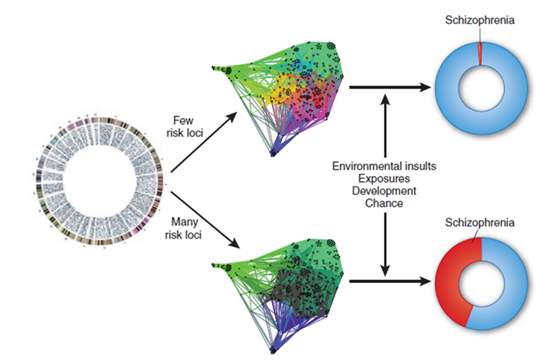
Polygenetic risk scores
How many risk genes you have - high polygenic risk score means that you have a lot of inherited genes
If we can identify genes associated with schizophrenia – look at what they do to piece together what goes wrong
Diseases are usually either genetic or sporadic – schizophrenia can be both so important to understand the mechanism of schizophrenia
1000s of risk loci but some stand out
DISC1 – scaffold protein associated with dopamine homeostasis
COMT – enzyme that breaks down dopamine
Neuregulin – signalling protein involved in cell proliferation and differentiation
These give clues that dopamine and synaptic transmission are involved in the pathology
COMT, DISC-1 and neuregulin are expressed during development.
Suggests that during development and developmental pruning (4-5 years and 11-13 years) may play a role
Psychosocial
important because the environment is important for precipitating the illness —> could be involved in the “second hit” of the 2 hit model
difficult to measure as it's hard to quantify, hard to control and is subjective —> hard to determine what is a stressor for some people
relapse rate is higher in “emotionally charged home environment
we need to look for other ways of measuring stress that’s more measurable – can look for a stress biomarker
age matched, gender matched inviduals with schizophrenia and without and exposed individuals to stressful paradigms and asked them about their childhood (E.g: whether they were physically, emotionally or sexually abused). The biomarker they then measured is salivary cortisol —> a more objective mechanism to measure psychosocial stress. In normal people when they’re exposed to a stressful situation they will respond with a cortisol spike, but in schizophrenics, there's a blunted cortisol response.
Cytoarchitectural abnormalities
dramatic pruning which does not occur in schizophrenics (also doesn’t occur in other psychiatric conditions)
causes increased sensitivity to noises (evidence of this is reduced paired pulse inhibition) and may explain the auditory hallucinations
generally don’t see this in imaging scans, the best you would see is decreased volume of the temporal lobe and slightly enlarged ventricles —> not a very good neurological substrate for disease as this is a non-specific indicator of white matter wastage.
Viral infection
if individuals born in late winter or spring with mothers who had experienced a viral infection in the second trimester, there was a greater risk of schizophrenia
may not be a great association as its more to do with when she was infected with the virus rather than the virus.
It could be that the viral infection subsequently impacts neurodevelopment:
epigenetic mechanism through hypermethylation of promoters such as DNMT1 which results in altered expression of schizophrenia candidate genes
Increased levels of maternal cytokines IL1beta, IL6 and TNF alpha that regulate normal brain development —> abnromal cortical development
could be effects on dopamine transmission
or by increasing the polygenetic risk score.
Summary of development theory
Multiple causes of schizophrenia and there are many routes that take you there
Evidence discussed supports the developmental theory of schizophrenia which states suggests a 2 hit model requiring neurodevelopmental changes, followed by an environmental stressor later in life to precipitate the disease.
First disturbance occurs during development and is caused by inheritance of genetic factors (high GRS) and viral infection which may alter gene expression
Evidence of this neural priming can be observed congentially or in the adult brain with subtle cytoarchitectural abnormalities
Finally, requires environmenal influence later in life to precipitate the disease —> implication of psychosocial
Sites of dysfunction in the brain
limbic regions
cerebral cortex
dorsolateral PFC
basal ganglia
Limbic regions
Found in temporal cortex
Limbic structures appear to show increased activity during auditory hallucinations
In schizophrenic patients: enlarged ventricles and decreased size of the lobe
Involved in processing auditory information and deciding what to do with it – either execute response or file it away in long term memory
Excessive activity means that information is being overprocessed in schizophrenia —> leading to auditory hallucinations
Sites of dysfunction in the brain
limbic regions
cerebral cortex
dorsolateral PFC
basal ganglia
Cerebral cortex
Dysfunction in the dominant cerebral hemispheres
Left hemisphere specialises in verbal function – in normal individuals there’s increased activity in left hemisphere during verbal tasks —> dominance in one brain region during a task is called lateralisation
Lateralisation disrupted in schizophrenia
Split brain patients is where there’s no communication between the left and right hemisphere
Sites of dysfunction in the brain
limbic regions
cerebral cortex
dorsolateral PFC
basal ganglia
Dorsolateral PFC
Within PFC is the ventral attention network – region where there’s excessive cortical thickness
PFC broadly controls complex cognitive behaviours
Social inhibition – essentially manners, hypoactivity in schizophrenics may lead to social inappropriateness
Decision making
Reasoning
Personality expression
Ventral attention Network can filter out unimportant stimuli
People with reduced activity in PFC (schizophrenics) have an inability in controlling these cognitive processes which explains dysfunctions in behaviour
Sites of dysfunction in the brain
limbic regions
cerebral cortex
dorsolateral PFC
basal ganglia
Basal Ganglia
Movement initiated in the frontal cortex must go through the basal ganglia where it gets fine tuned before you see the output
Has a role in the execution of movement
People with schizophrenia don’t usually display movement disorders but all movements must be “allowed to happen” by the basal ganglia. In instances of schizophrenics the basal ganglia is allowing movements which would normally not be allowed —> can explain the manifestation of some symptoms.
Plays a role in emotional processing as it sends projections up to the cortex to reward or aversive stimuli regions in frontal cortex, which allows you to process emotions
Emotional processing is impaired which means that the ability to feel happy or sad etc. is pronounced
Current understanding
Genetic susceptibility is involved but environment can modulate expression
Can explain positive symptoms in the temporal lobe
Can explain negative symptoms in the prefrontal cortex
problems with treatment
Different receptors, different neurotransmitters in different regions of the brain etc. —> cant treat all symptoms with the same approach
Multi-circuit approach required
Lots of emphasis now placed on regional changes
Dopamine (and glutamate) implicated in basal ganglia
Dopamine (glutamate and GABA) implicated in the lower frontal cortex. Dopaminergic neurons which originate in basal ganglia go up to frontal cortex
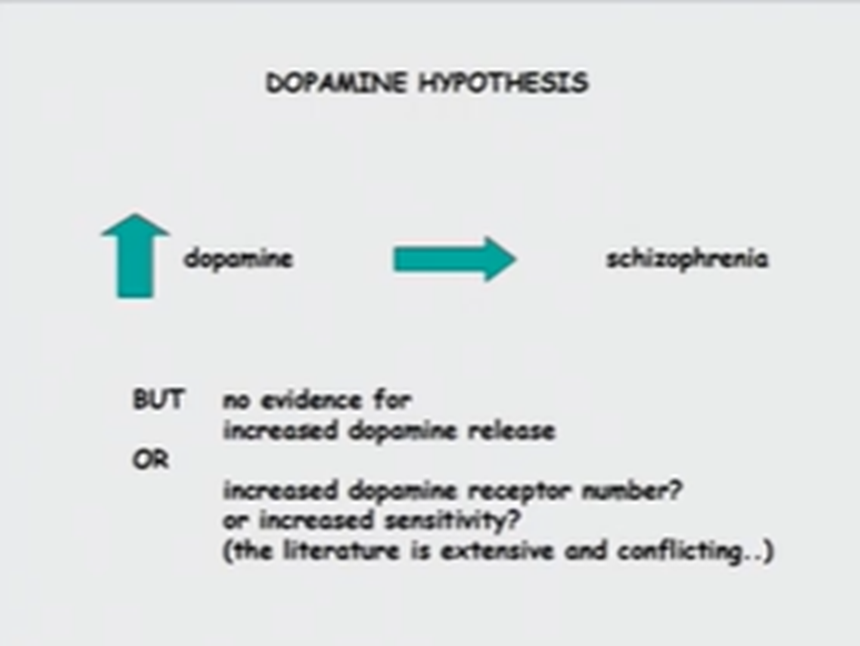
Neurochemical basis of schizophrenia - dopamine hypothesis
Suggestion that there's excessive dopamine transmission, which is an explanation of why you might have schizophrenia
Evidence which implicates dopamine as a causative agent of schizophrenia:
genes implicated in dopaminergic transmission contribute to risk scores
reserpine (depletes dopamine by blocking MAO uptake transporter) is an antipsychotic
amphetamine increases dopaminergic transmission and causes schizophrenia like symptoms in succeptible individuals (people who have genetic risk factors but not necessarily over the “stress threshold”)
L-DOPA precursor for dopamine (elevated dopamine) and triggers psychotic episodes. Difficulties with treating Parkinsons is not inducing psychosis.
Chlorpromazine
DAT knockout mice have prepulse inhibition deficits which can be rescued by administering DA antagonists.
Chlorpromazine
Revolutionised schizophrenia treatment
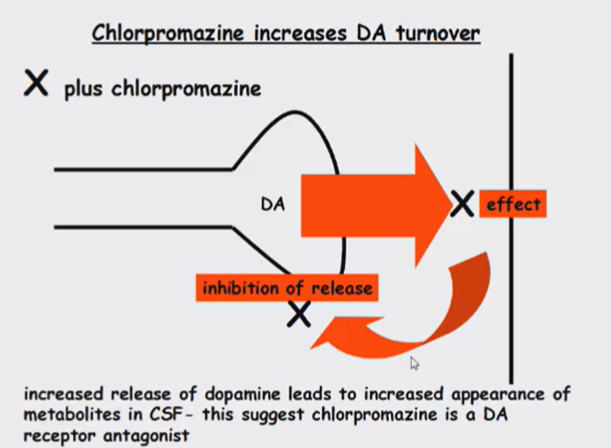
Dopamine receptor antagonist and works in 2 ways
Bind to dopamine receptor antagonistically
Increased turnover of dopamine
The stronger the binding to the D2 receptor, the greater the antipsychotic activity of a drug
Explaination for Schizophrenia
Therefore, we can explain the symptoms of schizophrenia due to excessive dopaminergic transmission feeding into the brain regions associated with social inhibitions and filetring out unimportant stimuli
But there's a multilayered explanation: Cytoarchitectural changes in the brain, which “prime” the brain for the even the small amount of environmental stress to trigger an increase in dopamine —> triggering a psychotic episode
But we can't assume that the dopamine hypothesis is the only explanation
Limitations to this hypothesis:
We need to see if teres increased dopamine release in brains of schizophrenics
No consistent evidence for increased dopamine release. Can be measured with CSF samples but it's unlikely that if someone is having a psychotic episode that they’ll be unlikely to give a CSF sample, so must be taken with a pinch of salt. As dopamine is turned over so quickly timing of samples very important
Studying the dopamine hypothesis
Dopamine hypothesis could be true but there could be many levels of increased dopamine levels
Increase in levels of dopamine – can be measured with CSF samples
Increase in binding of dopamine receptors
Decrease in clearance of neurotransmitter
Increase in receptor levels – studied in post-mortem brain
CSF samples
Dopamine metabolites found in CSF
However, if patients are having a schizophrenic episode, are unlikely to give a CSF sample
Difficult to do this as after the patient has calmed down, the dopamine levels have decreased
also doesn’t tell us anything about concentration of receptors or affinity of receptors
Post mortem brain
Can measure the levels of dopamine receptors in the post-mortem brain
Shows an increase in D2 receptors in postmortem brain
However, if these people have been treated with drugs, the increase in these receptors could be due to a homeostatic mechanism involving compensatory upregulation of dopamine receptors in response to you blocking dopamine receptor signalling (negative tolerance).
Wouldn’t be able to tell if the increase in D2 receptors is due to schizophrenia or due to treatment
PET
Ligand bound to a label using PET imaging to identify where D2 receptors are
can do these measurements in drug-naive patients
These patients do not show consistent increased levels of D2
However, just because these patients don’t have increased receptor levels doesn’t disprove the dopamine hypothesis, as we don’t know how potently the dopamine binds to these receptors
Also don’t know how much is released and how quickly its cleared
Dopamine hypothesis summary
Discrepancies
In depression, individuals with reduced Nadr signalling (characteristic of depression) if you give them drugs that increase this signalling you don’t show an immediate change in symptoms.
Like depression, with schizophrenia if you give them drugs which block dopaminergic transmission, does not cause an immediate effect
Implies that there are long-term changes in dopaminergic pathways that underpin the therapeutic effect
Requires chronic administration to see an effect —> long-term consequences as a result of blocking that receptor
This could be due to change in sensitivity of receptors, number of receptors etc.
May be change in signalling molecules in a cell
May be the overall compensatory effect on the signalling pathway
The fact that we see a beneficial effect is encouraging, but we need to understand the mechanism
Mechanism
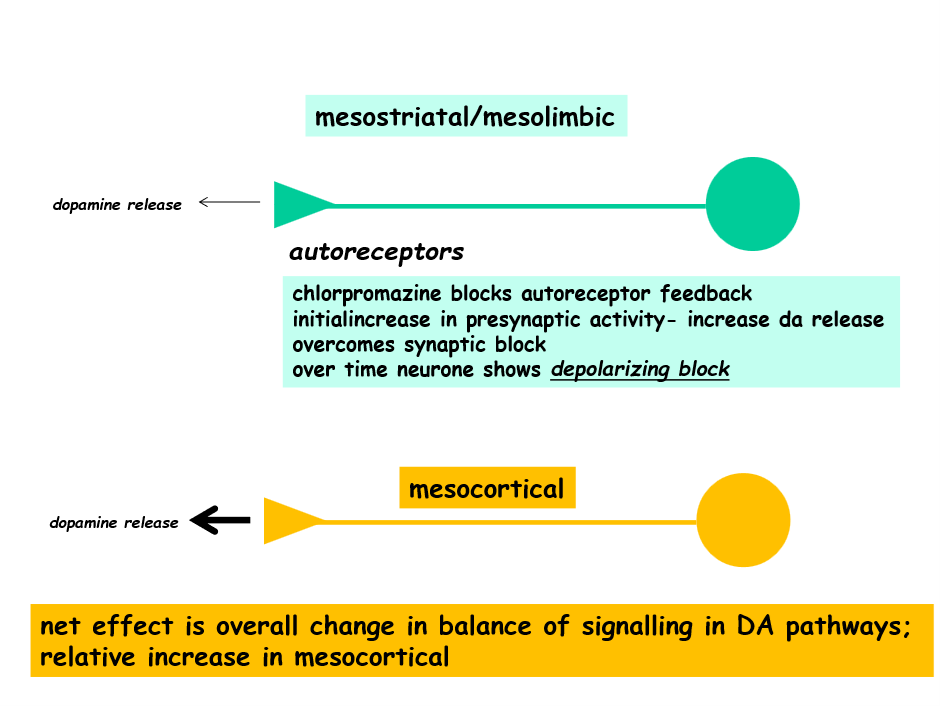
Chlorpromazines primary mechanism of action is binding antagonistically to the D2 receptors on the post synaptic nerve terminal, however, it also antagonistically binds to autoreceptors on the presynaptic nerve terminal.
When bound to presynaptic receptors it causes an initial increase in DA in the synaptic cleft (due to blocking a block leading to increased DA release)
However, over time the post synaptic receptor blockage dominates leading to compensatory changes in signalling.
In schizophrenics, there tends to be a hyperactivity in the mesolimbic dopamine pathway, responsible for positive symptoms (VTA->Nacc) whereas there’s a hypoactivity in the mesocortical pathway, responsible for negative symptoms (VTA —> PFC)
Due to more dense D2 receptor populations in the Nacc and baseline activity of receptors differing, chlorpromazine has a greater affect on the mesolimbic pathway
This causes a change in balance of the two dopamine pathways
Typical antipsychotics (neuroleptics)
Typical
Old fashioned drugs originally found to be antipsychotics
Such as: phenothiazines, thioxanthenes and butyrophenones
Had limitations as they were only effective against positive symptoms in some patients
Had lots of side effects such as weight gain, sedation (were they effective against positive symptoms or just sedating them), postural hypotension
Some of these side effects may be due to binding to adrenergic and histamine receptors (off-target effects occurring when it binds to a different receptor at lower affinity)
Could end up with Parkinsonian symptoms like tardive dyskinesia (irreversible symptom whetre you have repetitive meaningless movement) —> The substantia nigra the area of the brain responsible for initiating movement using dopamine but the drug does not discriminate between dopamine receptors in substantia nigra and the PFC.
Atypical antipsychotics (neuroleptics)
Atypical
Wanted to create a drug which has a higher affinity for dopamine receptors in a specific region —> newer second-generation drugs
Had less sedation and reportedly lower incidence of movement disorders
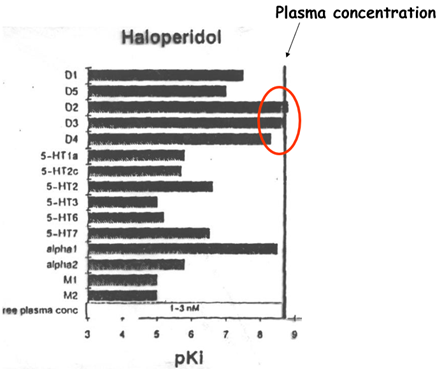
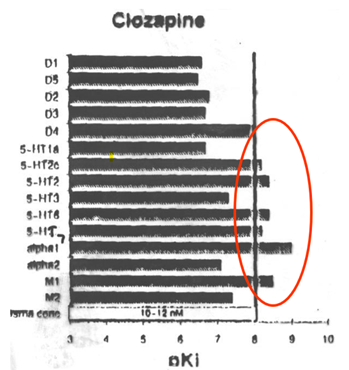
Examples: clozapine, quetiapine, olanzapine, risperidone, aripiprazole, asenafine, paliperidone, haloperidol
Has a receptor profile which means that they are more specific to the dopamine receptor —> high affinity for D2 and D3 receptors implicated in regions of the brain you want to inhibit.
On the other hand the Clozapine (an atypical antipsychotic) was acting on the adrenergic and histaminergic receptors which is why it had a lot of the side effects.
Things to consider when designing a drug
Specificity of dopamine receptors in a specific brain region
Pharmacokinetics —> such as how often you need to take the medication, for schizophrenia where administration is chronic, you ideally don’t want the drug to have to be taken frequently to increase compliance
How long it takes to cause effect
Side effects and toxicity
Cost
Drug interactions
Blood-brain barrier permeability —> need the drug to efficiently penetrate the blood brain barrier efficiently
Animal models for schzophrenia
How do we generate animal models
We know that in schizophrenic individuals one of the causes is increased dopamine transmission, so we want to recreate the causes in animals
To simulate this we can look to see if you can insert risk factor genes such as
DISC-1
COMT
COMT genes
Individuals with velocardiofacial syndrome have a 1.5-3Mb deletion in chromosome 22 (deletion of 30 genes) which greatly increases risk of schizophrenia
In schizophrenia populations there are far higher incidences of this 30 gene deletion compared to the rest of the population
COMT (catchol-O-methyl transferase) genes involved in the breakdown of dopamine —> breaks catechol down into O-methyl catechol
2 COMT alleles in humans: COMT valine 108, COMT methionine 108 (less stable enzyme and low activity variant)
Individuals with COMT methionine 108 should in theory have higher levels of synaptic dopamine, and greater incidence of schizophrenia
Can insert these into animal models then screen these animal models to see if they express schizophrenic symptoms
DISC-1
Gene initially identified in a family with high incidence of psychiatric disorders, particularly schizophrenia
Believed to be as a result of chromosomal translocation —> during meiosis you have breakages which allow for recombination
Can look at the interactome of DISC-1 showing all the proteins it interacts with
Generally DISC-1 has a role in neural growth, neural migration and synaptic regulation
Generating animal models
Genetic induced models
How can we use these new models to help understand, create therapies for and model schizophrenia?
Gene knockouts, however, we need to know if this gene is over or under active in schizophrenia
If its overactive in schizophrenia and we knock it out it wont model schizophrenia —> need to use expression activators to control expression of the gene
Or you can look at which version of the gene that is most prevalent in schizophrenics
in the example of the COMT gene this is the mathionine 108 and its not the over or under expression of this, just the presence.
Models for changes in brain architecture
In parts of the brain where there’s reduced activity we can capitulate lesions in this area to see how the animal is affected
Models for Developmental changes
Postnatal isolation - when the pup and mum are separated right after birth can recapitulate the environmental stress experienced by schizophrenics
Drug induced models
Can give the animals psychotics which mimic a psychotic episode
Prepulse inhibition (PPI)
prepulse inhibition of startle is a cross species measure that refers to the ability of a non-startling “prestimulus”to inhibit the response of a second startling stimulus
The initial weak sensory signal reduces the body’s startle response to a subsequent strong startling sound. Startle response is measured by comparing the amplitude of EMG response of hearing the startling sound alone and in comparison to when its preceded by a non startling prepulse.
However, because reduced PPI is caused by the balance of neurotransmitters, underlying circuitry and genetic factors, PPI deficits also observed in other neuropsychiatric disorders such as tourettes, OCD, adults with autism and manic bipolar pts —> might not be a good model for schizophrenia specifically
has a genetic component and is a useful psychophysiological study to use in mouse and human models as well as a potential vulnerability marker for psychosis
Causes of reduced PPI
Excess of dopamine in the temporal lobe causing the overprocessing of information
DA agonists are able to reproduce PPI reduction wheras antipyschotics partially normalise it
reduced NMDA receptor function, particularly on GABAergic inhibitory neurons.
loss of dysfunction of GABAergic neurons
How can we use these animal models?
Expose the rodents to stressors
Can measure this with salivary cortisol (biomarker of stress) —> better than generic stressors as stress is objective
What are some of the associated problems?
So many symptoms of schizophrenia which are human specific, and we have no way of determining if these symptoms occur in animals and how we measure this.
With other neurological conditions, like depression, they exhibit behaviour such as sickness behaviour which makes it slightly easier to observe. With schizophrenia this is more difficult
For positive symptoms the only things we can measure is increased locomotor symptoms. Even still this is a pseudo measure of symptoms.
Negative symptoms are much more difficult to quantify. Only measure we currently have is reduced social interaction