Common Signaling Pathways in Mechanotransduction
1/106
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
107 Terms
Cellular mechanotransduction
The process by which cells convert mechanical signals into biochemical responses.
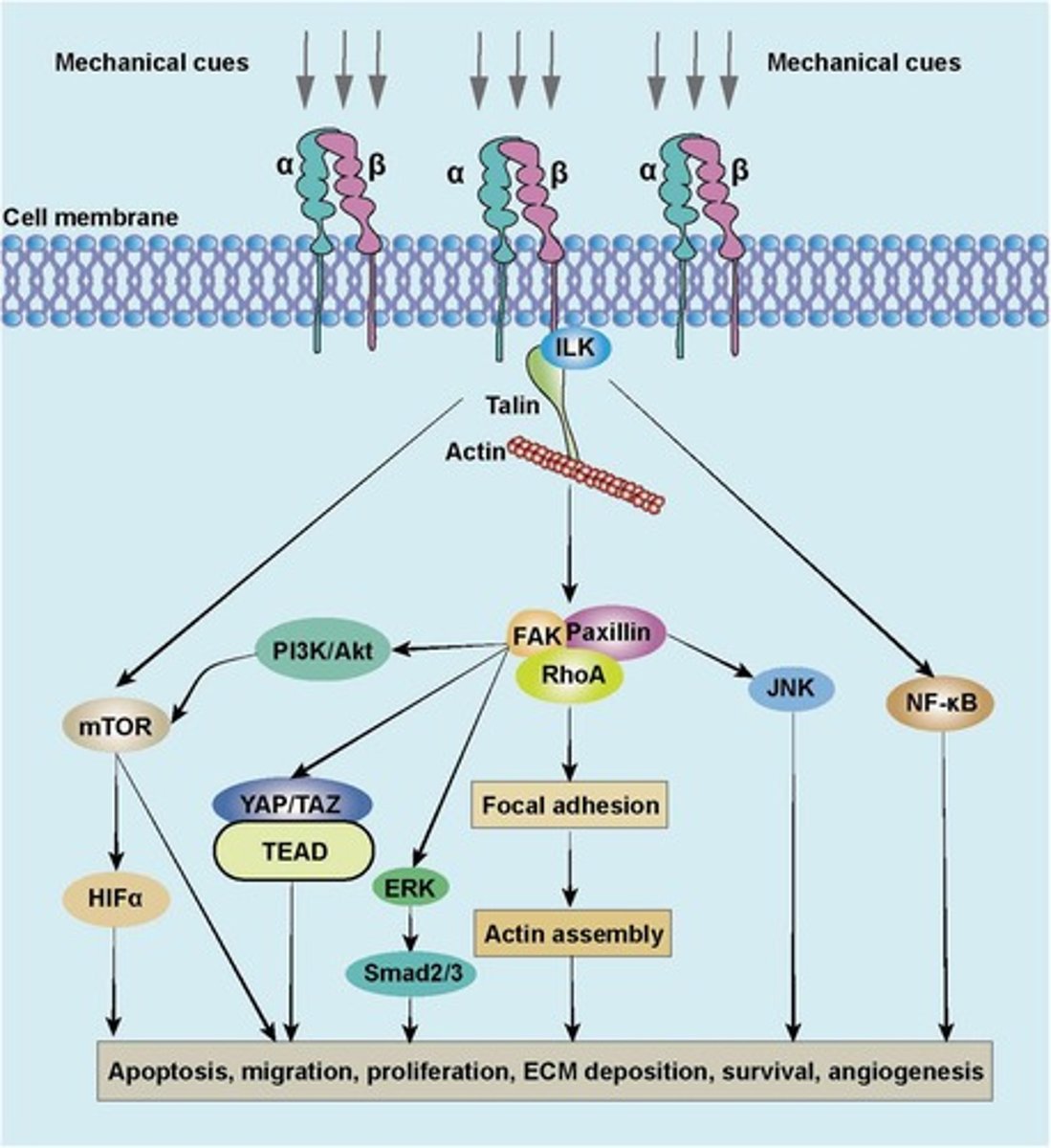
Focal Adhesion Kinase (FAK) Pathway
Converts mechanical forces at the extracellular matrix (ECM) into intracellular signaling cascades.
Focal Adhesions (FAs)
Mechanosensors linking integrins to the cytoskeleton.
FAK Activation
Integrins cluster in response to ECM stiffness, activating FAK autophosphorylation at Y397.
Phosphorylated FAK
Recruits Src kinase, amplifying downstream signaling.
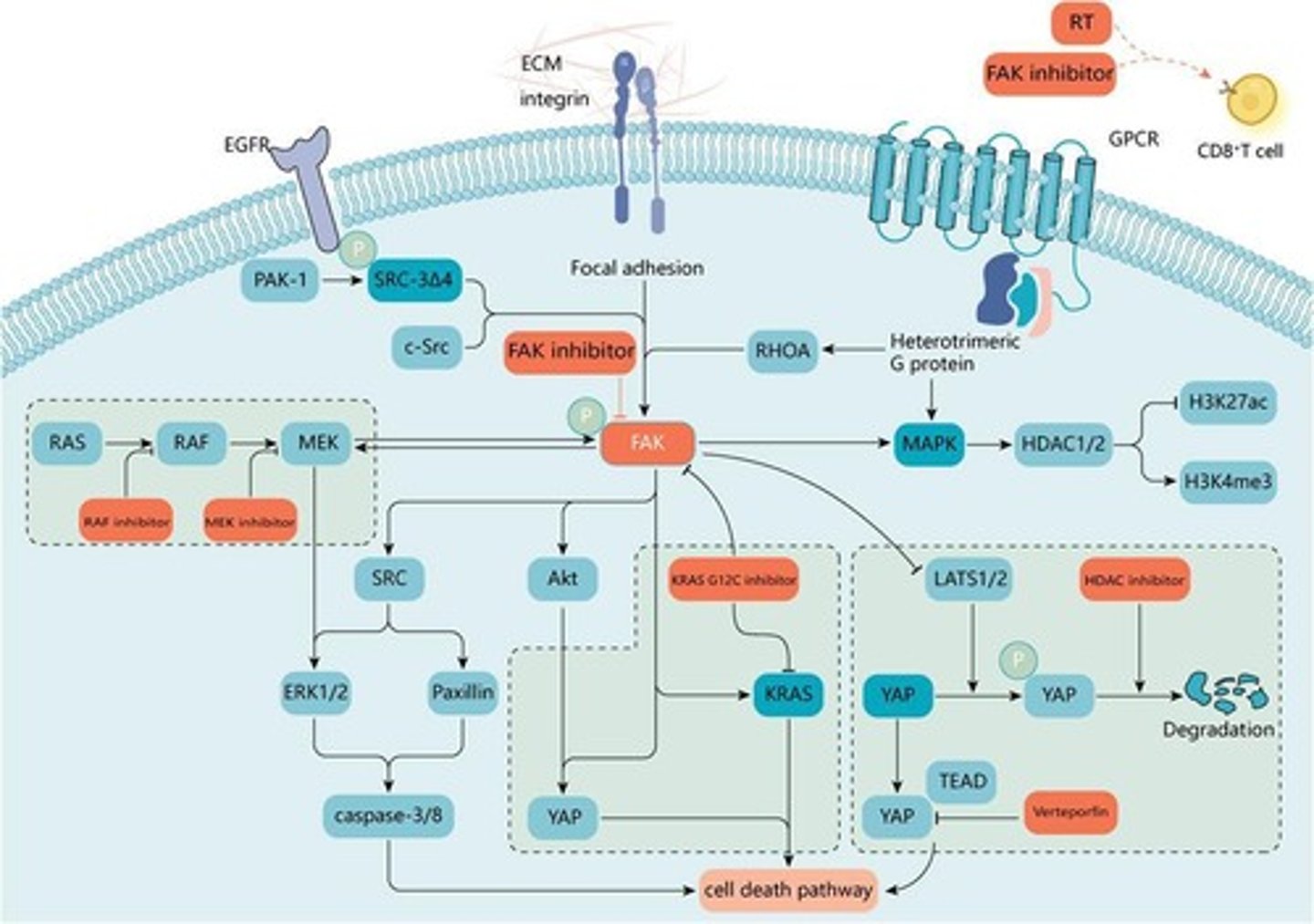
FAK-Ras-MAPK pathway
Promotes cell survival and proliferation.
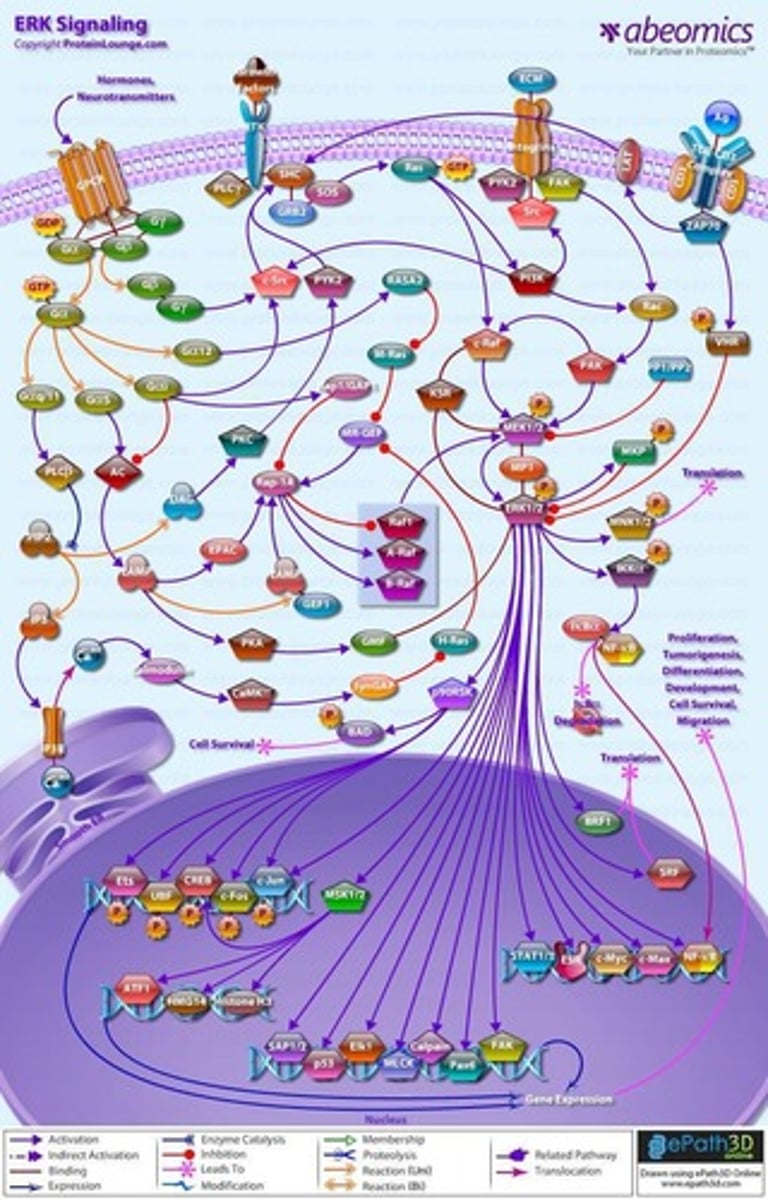
FAK-PI3K-Akt pathway
Regulates cell adhesion and migration.
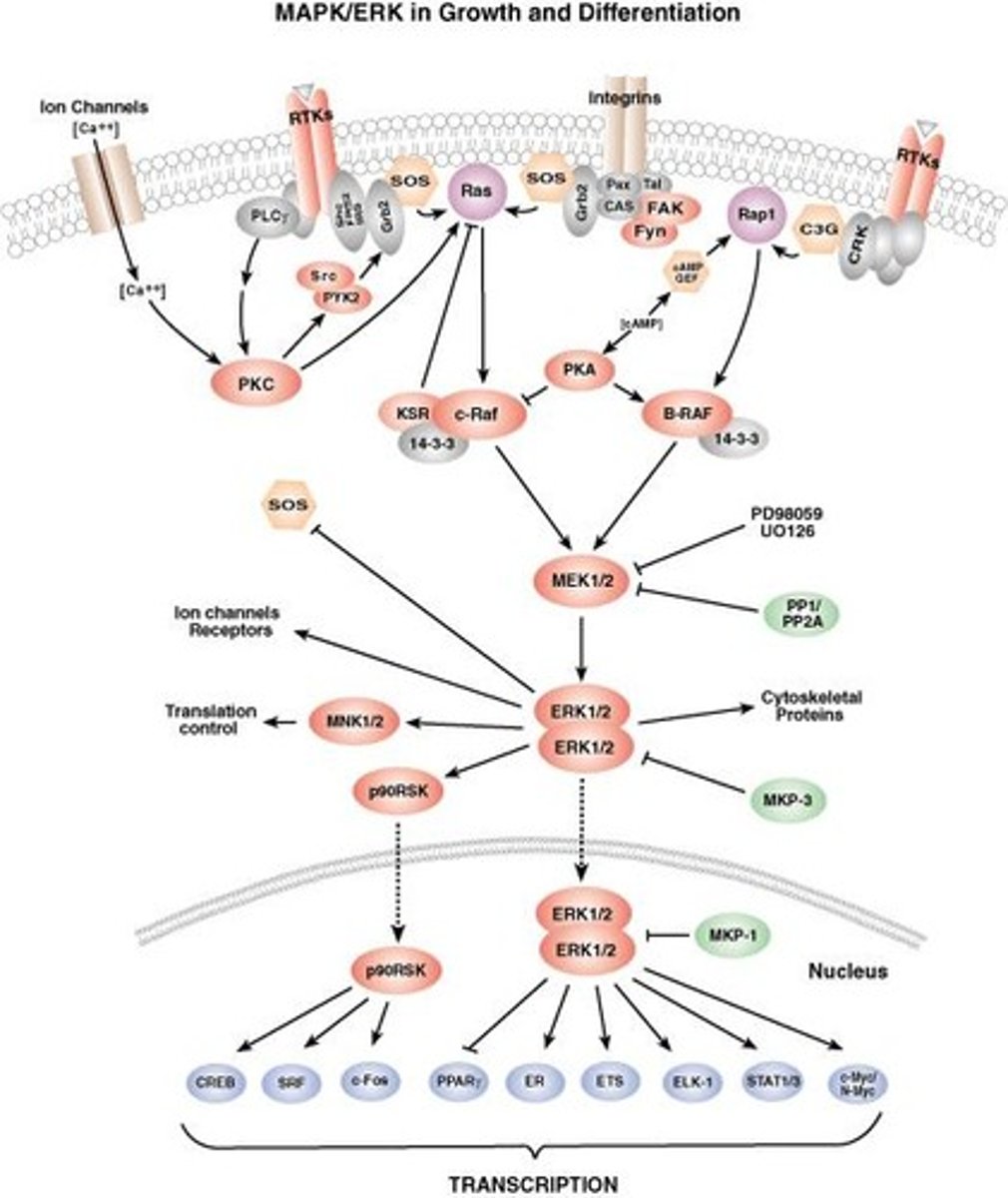
FAK-YAP/TAZ crosstalk
Activates YAP/TAZ via cytoskeletal remodeling.
Mechanotransduction Relevance in Cancer
Elevated FAK signaling enhances metastasis by promoting cell invasion.
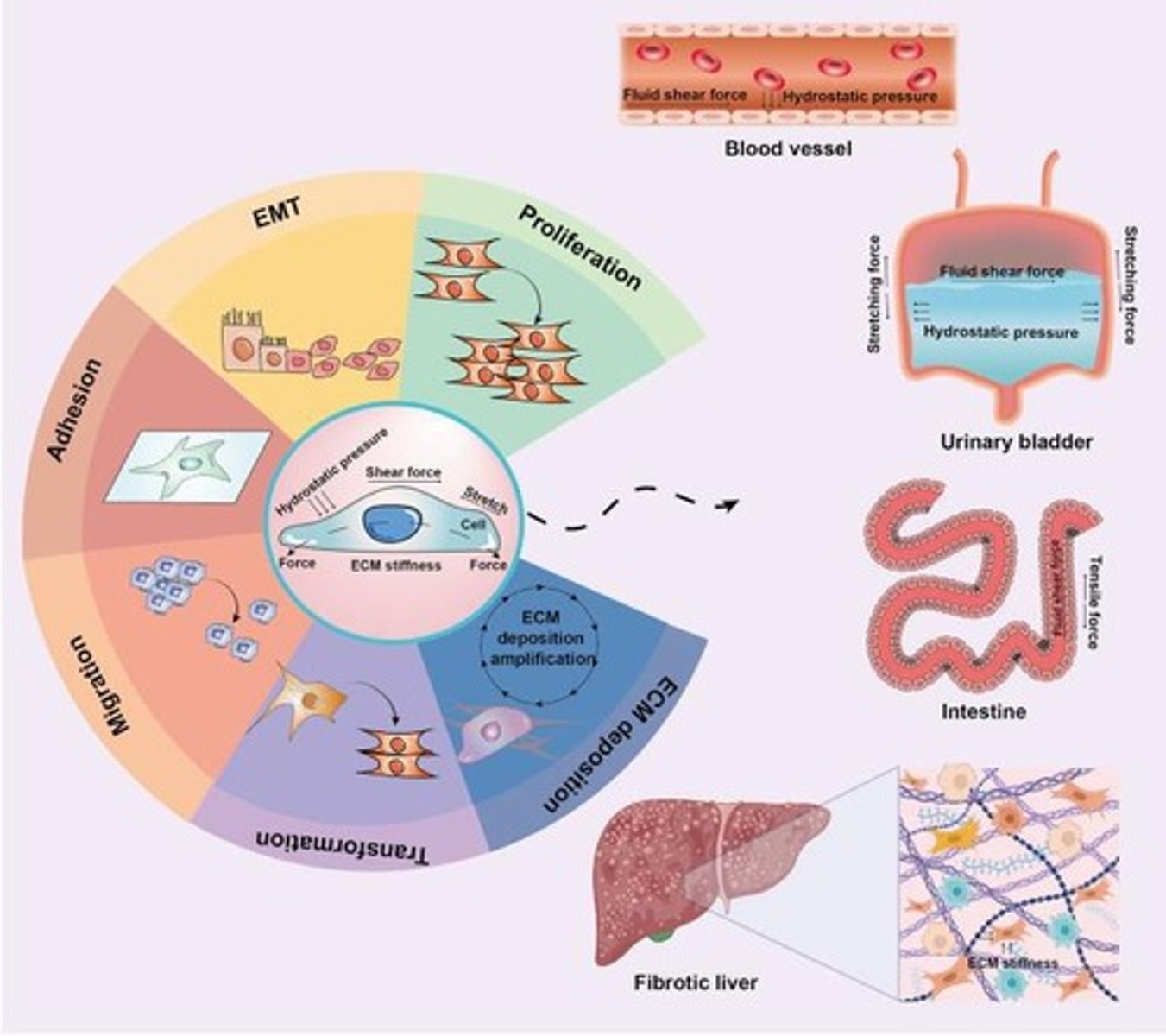
Mechanotransduction Relevance in Fibrosis
FAK signaling contributes to myofibroblast activation in stiff ECM.
YAP/TAZ (Hippo) Pathway
Transcriptional co-activators that respond to mechanical cues, controlling gene expression.
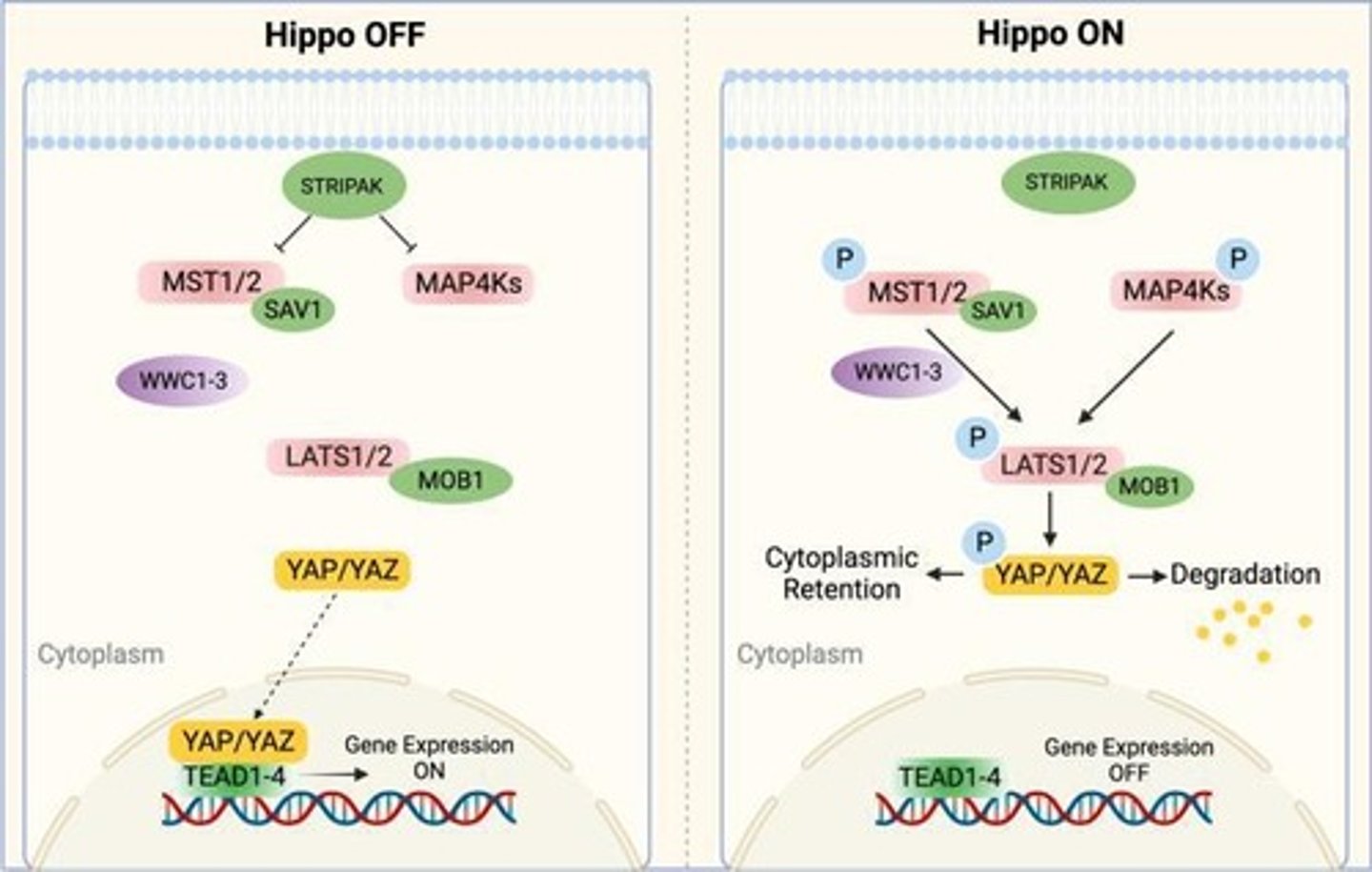
Mechanosensitive Activation of YAP/TAZ
Under soft ECM conditions, YAP/TAZ are phosphorylated by LATS1/2, preventing nuclear localization.
YAP/TAZ translocation
Under stiff ECM conditions, actin cytoskeleton tension inhibits LATS1/2, allowing YAP/TAZ to enter the nucleus.
YAP/TAZ-TEAD interaction
Activates genes related to proliferation, survival, and ECM remodeling.
Crosstalk with β-catenin
Enhances stem cell differentiation.
Mechanotransduction Relevance in Cancer (YAP/TAZ)
YAP/TAZ is a key driver of tumor growth and metastasis in stiff microenvironments.
Mechanotransduction Relevance in Fibrosis (YAP/TAZ)
Increased YAP/TAZ activity drives fibroblast-to-myofibroblast differentiation.
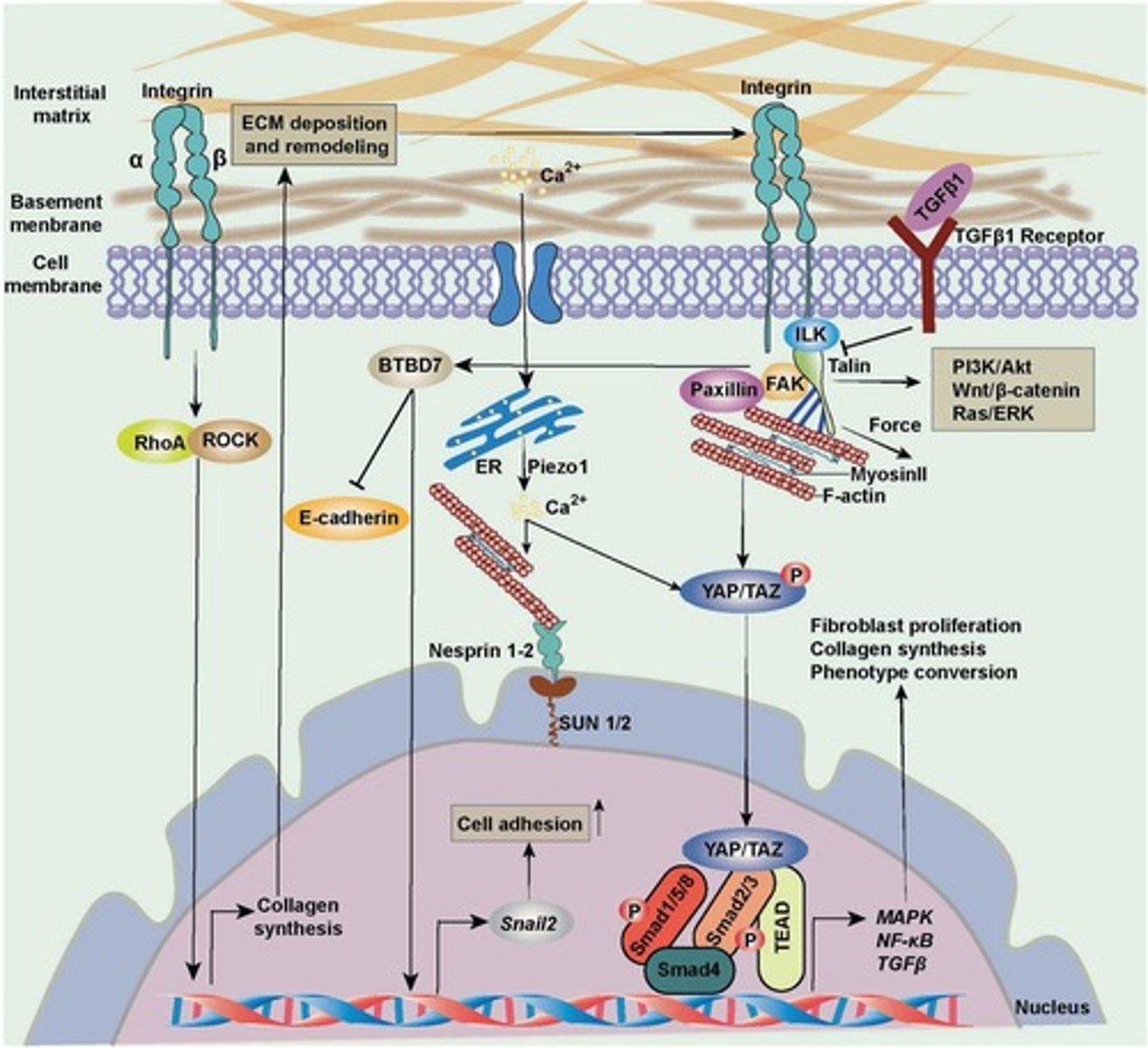
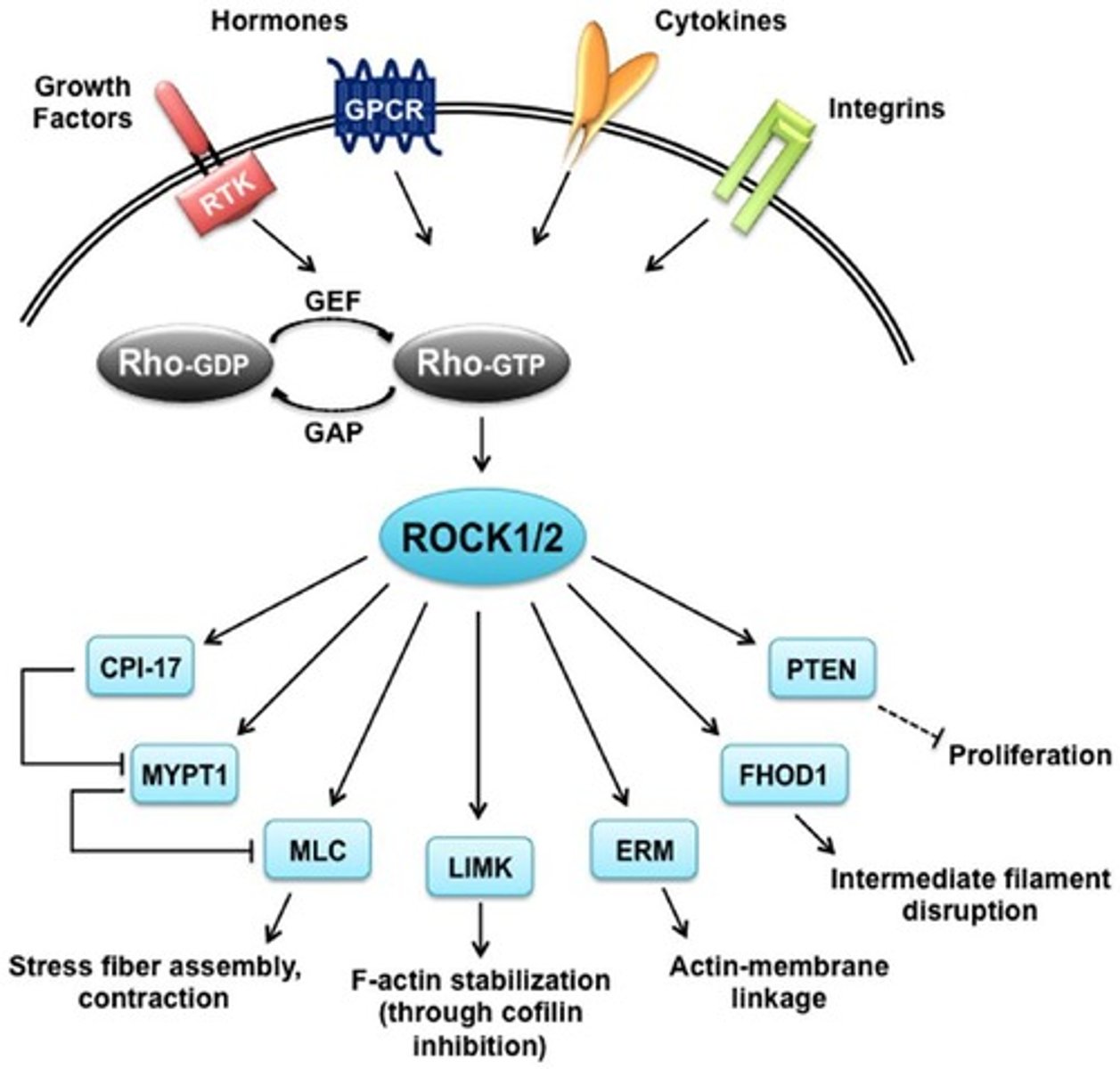
RhoA/ROCK Pathway
Regulates actin cytoskeleton organization in response to mechanical forces.
Activation Mechanism of RhoA/ROCK
Mechanical stress activates RhoA GTPase, which in turn activates ROCK (Rho-associated kinase).
ROCK phosphorylates MLC
Promotes actomyosin contraction.
Downstream Effects of RhoA/ROCK
Actin polymerization and stress fiber formation; enhances focal adhesion stability via vinculin and talin recruitment.
Mechanotransduction Relevance in Cancer (RhoA/ROCK)
Increased RhoA/ROCK signaling promotes cell migration and metastasis.
Fibrosis
RhoA/ROCK activation increases myofibroblast contraction and ECM stiffening.
Function of Rho-Associated Kinases (ROCK)
Controls ECM remodeling and fibrosis through mechanotransduction.
Activation Mechanism of TGF-β
TGF-β is sequestered in the ECM and released by mechanical forces (e.g., ECM stiffening).
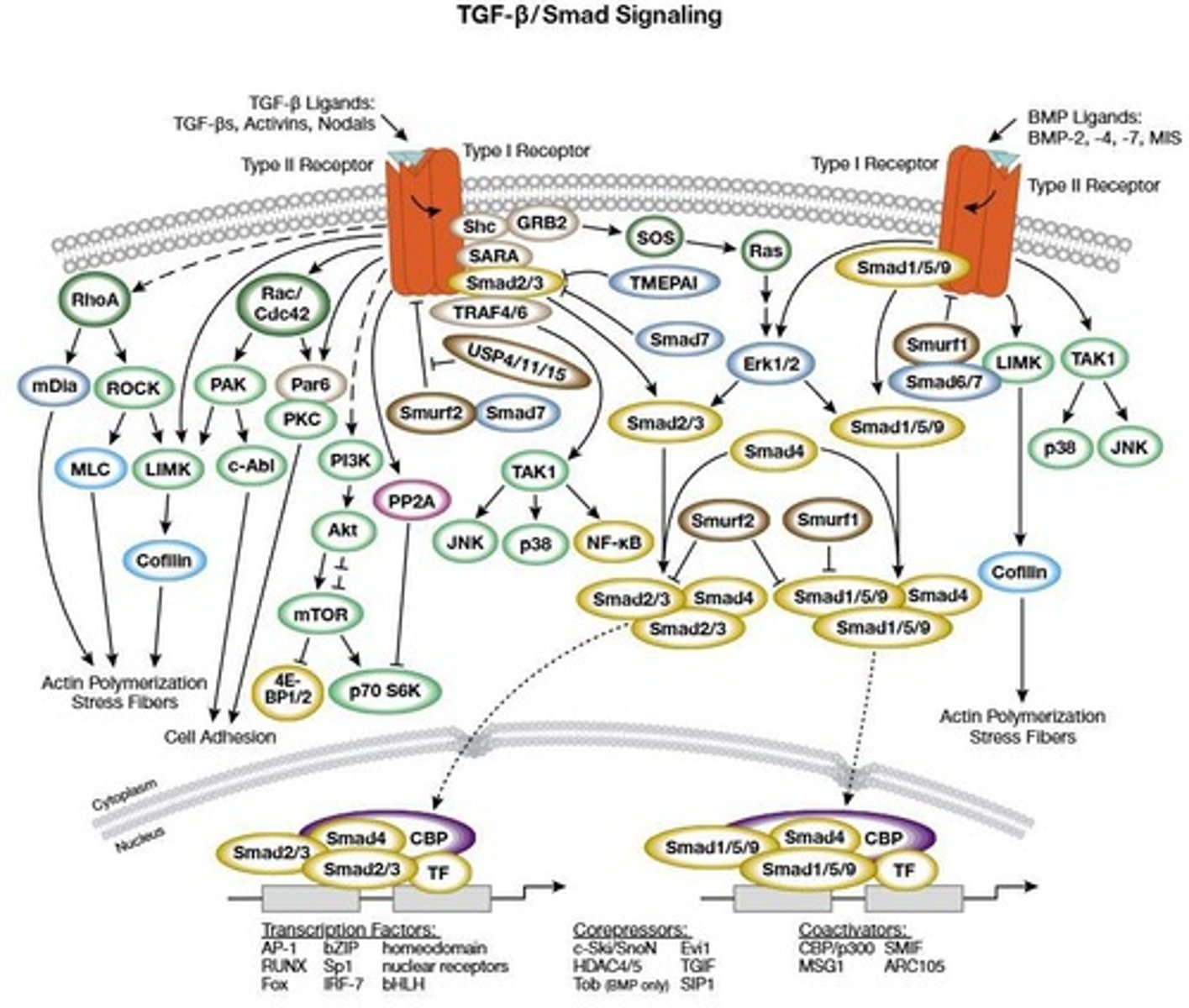
TGF-β Receptors
TGF-β binds TGF-β receptors (TβRI/TβRII), leading to SMAD2/3 phosphorylation.
SMAD2/3 Phosphorylation
Phosphorylated SMAD2/3 complexes with SMAD4, translocates into the nucleus, and regulates gene expression.
Downstream Effects of TGF-β
Promotes fibrosis by upregulating α-SMA, collagen, and fibronectin.
Crosstalk with YAP/TAZ
Crosstalk with YAP/TAZ and β-catenin enhances pro-fibrotic signaling.
Mechanotransduction Relevance in Fibrosis
Excessive TGF-β/SMAD activation leads to myofibroblast differentiation and ECM deposition.
Mechanotransduction Relevance in Cancer
TGF-β plays dual roles, acting as a tumor suppressor in early stages and a metastasis promoter in later stages.
β-Catenin/Wnt Pathway Function
Regulates cell fate, differentiation, and adhesion in response to mechanical cues.
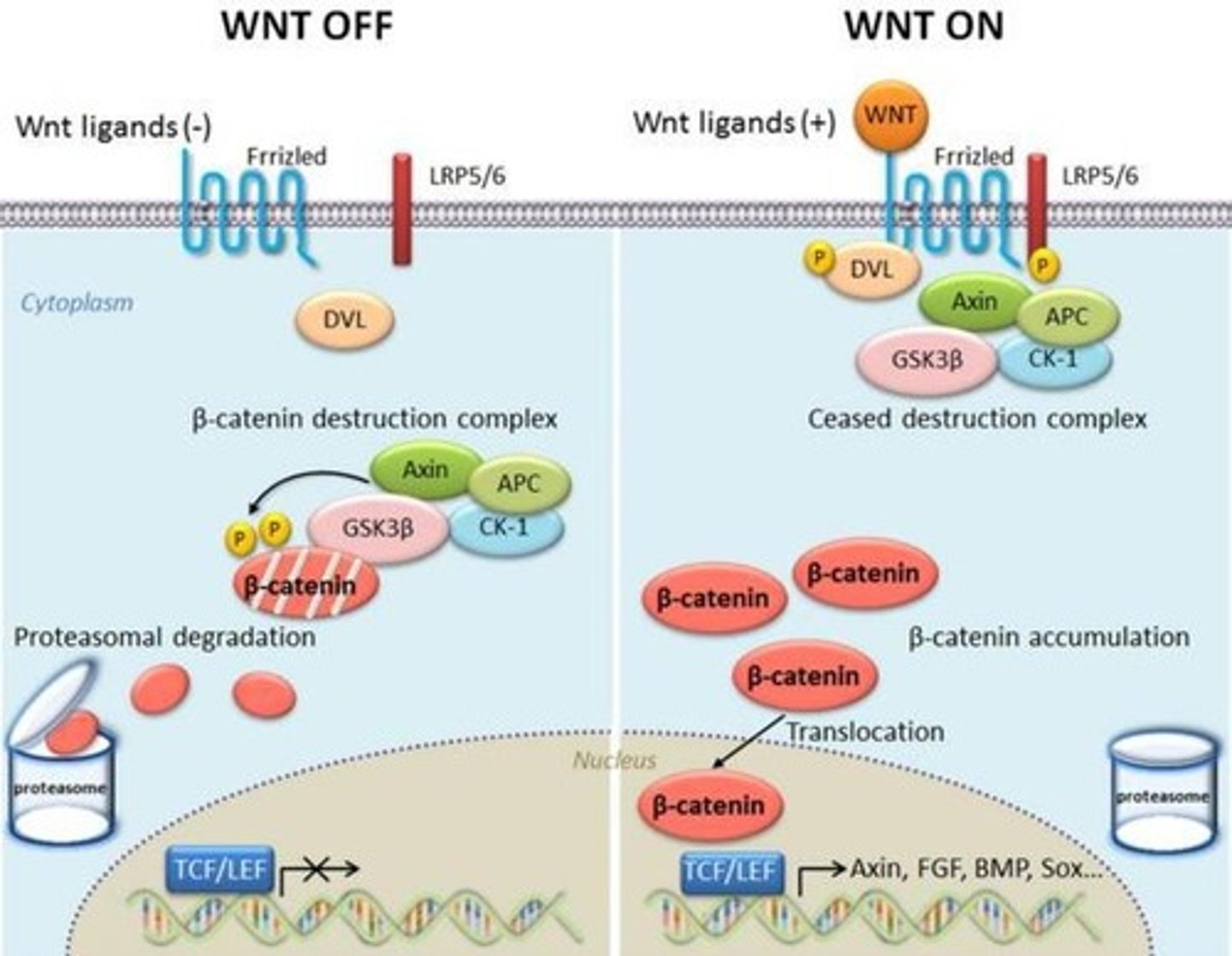
Activation Mechanism of β-Catenin
Under low mechanical stress, β-catenin is degraded by the Axin-APC-GSK3β complex.
High Mechanical Stress Effect
Under high mechanical stress, Wnt ligands bind Frizzled receptors, leading to β-catenin stabilization.
β-Catenin Accumulation
β-catenin accumulates in the nucleus and activates transcription factors (LEF/TCF).
Crosstalk with Mechanotransduction in β-Catenin
Integrins and cadherins regulate β-catenin stability in response to force.
Stem Cell Differentiation
Stiff matrices promote osteogenic differentiation via β-catenin.
Notch Pathway Function
Controls cell differentiation and fate decisions through direct cell-cell mechanical signaling.
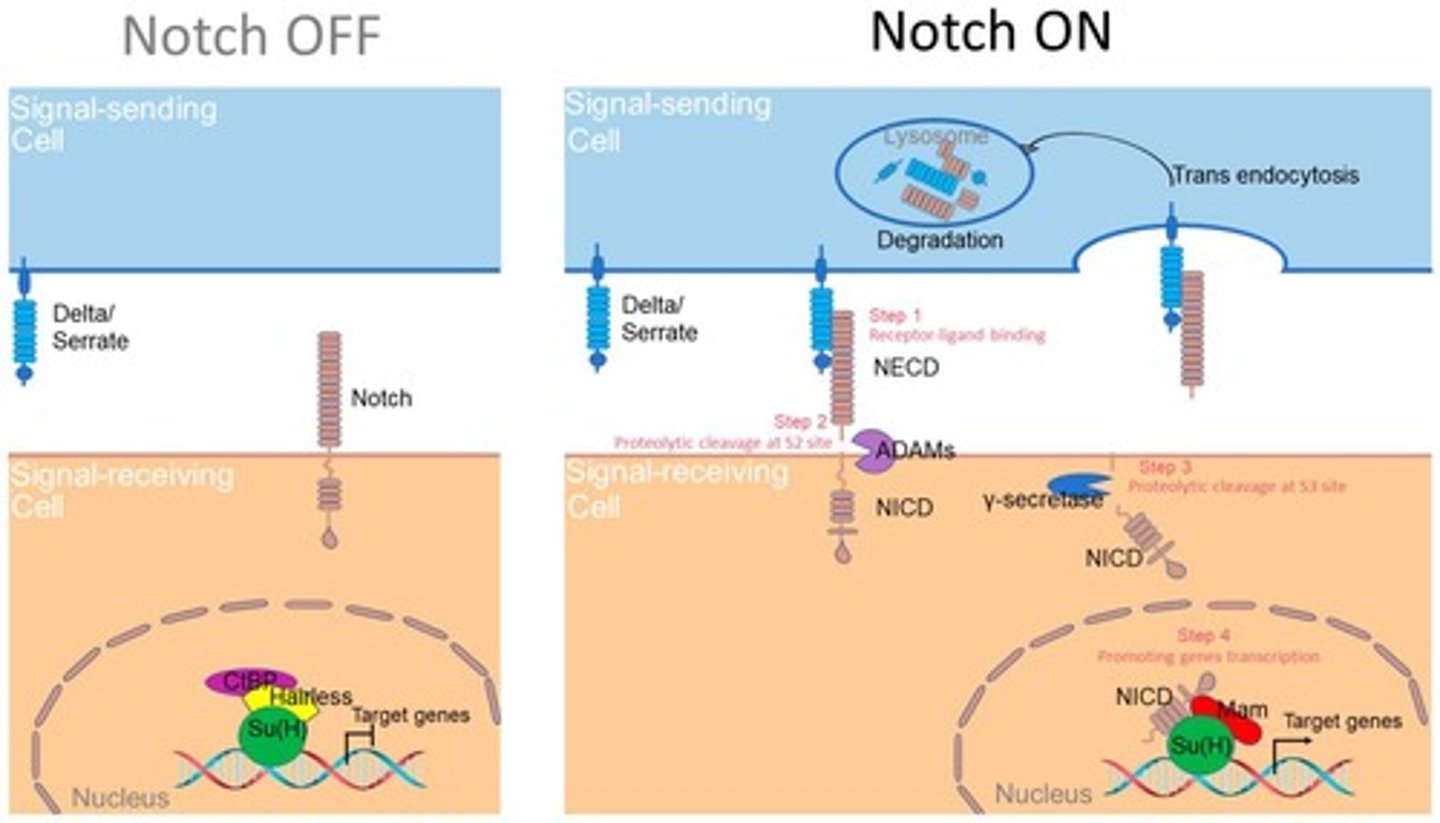
Activation Mechanism of Notch
Ligand-receptor interaction (Notch-Delta/Jagged) is mechanically regulated.
Notch Cleavage
Under force, Notch undergoes proteolytic cleavage, releasing Notch Intracellular Domain (NICD).
NICD Function
NICD translocates into the nucleus to regulate gene expression.
Crosstalk with Mechanotransduction in Notch
Integrins and cytoskeletal tension enhance Notch signaling.
ERK/MAPK Pathway Function
Mediates cell proliferation, differentiation, and survival in response to mechanical forces.
Activation Mechanism of ERK
Shear stress, ECM stiffness, and integrin activation lead to FAK-Src-Ras signaling.
Ras-GTP Activation
Ras-GTP activates Raf (MAPKKK) → MEK1/2 (MAPKK) → ERK1/2 (MAPK).
ERK Translocation
ERK translocates to the nucleus, activating transcription factors like c-Myc, AP-1.
Relevance of ERK in Cancer
ERK overactivation promotes tumor proliferation and drug resistance.
Fibrosis
ERK regulates fibroblast activation and ECM deposition.
FAK (Focal Adhesion Kinase)
Activated when integrins bind ECM proteins, triggering FAK autophosphorylation and recruiting Src kinase.
PI3K-Akt
A downstream pathway activated by FAK that is involved in survival.
Ras-ERK
A downstream pathway activated by FAK that is involved in proliferation and migration.
YAP/TAZ
Transcriptional control that regulates stiffness-dependent gene expression.
RhoA/ROCK
Controls actin tension and focal adhesions, involved in cytoskeletal dynamics.
TGF-β/SMAD
Promotes fibrosis and interacts with YAP/TAZ for ECM remodeling.
β-Catenin/Wnt
Stiffness promotes osteogenic lineage differentiation.
Notch
Regulates force-dependent differentiation and cell-cell communication.
ERK/MAPK
Regulates cell proliferation, differentiation, and survival.
Mechanotransduction
Integrates signals from ECM stiffness, shear stress, and integrins to activate gene transcription and cytoskeletal remodeling.
LATS1/2 kinase
Inactivated in a stiff ECM, allowing YAP/TAZ to promote proliferation and survival.
Actomyosin contraction
Stimulated by ROCK phosphorylation of myosin light chain (MLC), enhancing focal adhesion stability.
Integrins
Proteins that mediate cell-ECM adhesion and are involved in mechanotransduction.
Mechanical force
Stimulates RhoA activation, leading to cytoskeletal contraction.
ECM stiffness
Cells sense ECM stiffness through integrins and cytoskeletal tension.
Tumor cell invasion
Blocking integrins may be a better strategy than inhibiting FAK directly.
Scar tissue fibroblasts
Often have high RhoA/ROCK activity due to mechanical force responses.
Cell migration ability
Would be impaired if FAK is permanently activated, leading to no focal adhesions.
FAK inhibitor
Could affect cancer metastasis by inhibiting cell migration.
Soft ECM
Leads to YAP/TAZ phosphorylation and degradation, reducing proliferation.
Fibrotic diseases
Involve excessive tissue stiffening.
TGF-β
Sequestered in the ECM and released by mechanical stress or proteases.
SMAD2/3
Phosphorylated by TGF-β binding to TβRI/TβRII receptors.
SMAD4
Complexes with SMAD2/3 and translocates to the nucleus to activate fibrosis-related genes.
ERK
Activated by Ras, leading to cell division and survival.
c-Myc
Stimulated by active ERK to promote cell division.
AP-1
Activated by ERK to promote cell survival.
Notch signaling
Activated when Notch receptors interact with Delta or Jagged ligands.
YAP/TAZ
Nuclear translocation increases with stiff ECM, enhancing proliferation and survival.
LATS1/2
When activated in a stiff ECM, leads to decreased cell proliferation.
FAK
Promotes focal adhesion turnover and cytoskeletal dynamics.
Integrin clustering
When blocked, impairs FAK signaling and reduces cell adhesion, migration, and survival.
RhoA
Inhibition leads to breakdown of actin stress fibers and weaker adhesion.
ROCK
Increasing activity affects cell stiffness.
Mechanical stress
Can enhance Notch activation, leading to gene expression changes.
Tumor cell proliferation
Increases with hyperactive ERK.
Endothelial cells
Survive under high shear stress due to ERK activation.
Vascular graft
Mechanotransduction can be used to activate Notch signaling for endothelial cell differentiation.
Cell migration
Increases with permanently activated FAK.
Cancer metastasis
Reduced by FAK inhibitors preventing focal adhesion turnover.
TGF-β inhibitors
Might be used to treat fibrosis.
Shear stress
Can influence Notch activity in vascular endothelial cells.
Cell stiffness
Increases due to enhanced actomyosin contraction and focal adhesion reinforcement.
RhoA/ROCK
High activity in fibroblasts in scar tissue due to mechanical stiffness, activating RhoA/ROCK to maintain cytoskeletal tension and ECM remodeling.
TGF-β/SMAD
If TGF-β availability is reduced, SMAD2/3 phosphorylation would decrease, reducing fibrosis-related gene expression.
TGF-β/SMAD
TGF-β signaling would increase as mechanical stress enhances TGF-β release and receptor activation.
TGF-β/SMAD
TGF-β inhibitors may be used to treat fibrosis because fibrosis is driven by excessive ECM deposition via TGF-β signaling.
ERK/MAPK
If MEK is inhibited, ERK activation would be blocked, stopping downstream proliferation signals.
ERK/MAPK
If a tumor cell has hyperactive ERK, its proliferation rate would increase, as ERK drives cell cycle progression and survival.
ERK/MAPK
ERK activation helps endothelial cells survive under high shear stress by activating ERK via integrins.
Notch
If Notch signaling is blocked in a developing embryo, developmental defects would occur because Notch is essential for tissue differentiation and organogenesis.
Notch
Increased shear stress enhances Notch activation, promoting endothelial cell differentiation and vascular stability.