8th Grade Chemistry
0.0(0)
Card Sorting
1/60
Last updated 2:46 PM on 11/15/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
61 Terms
1
New cards
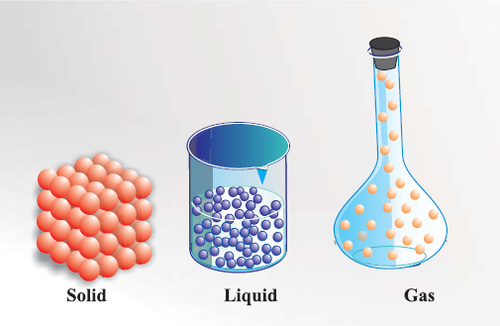
Matter
What everything is composed of
2
New cards
Atom
The simplest particle of an element
3
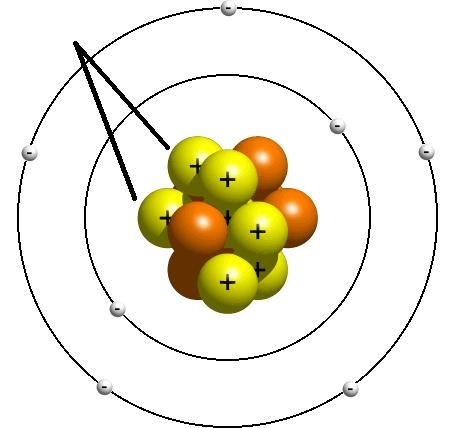
New cards
Proton
A subatomic particle that has a positive charge and that is found in the nucleus of an atom
4
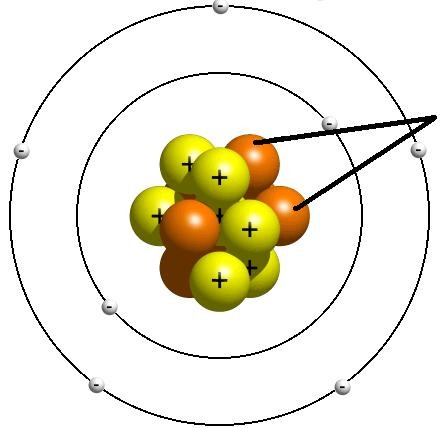
New cards
Neutron
A subatomic particle that has no charge and that is found in the nucleus of an atom
5
New cards
Electron
A subatomic particle that has a negative charge
6
New cards
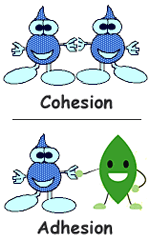
Cohesion
an attraction between molecules of the same substance
7
New cards
Surface Tension
A measure of how difficult it is to stretch or break the surface of a liquid
8
New cards
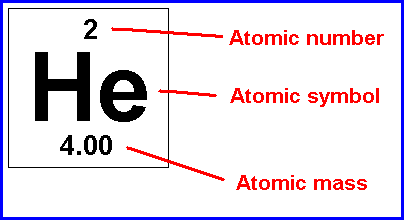
Element
A pure substance that cannot be broken down chemically into simple kinds of matter
9
New cards
A compound
What is a pure substance made up of two or more elements?
10
New cards
A molecule
_________ is the simplest part of a substance that still has all of the properties of that substance.
11
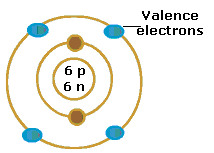
New cards
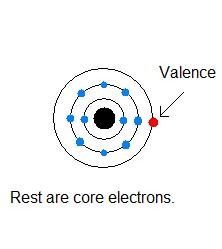
Valence Electron
Electrons on the outermost energy level of an atom
12
New cards
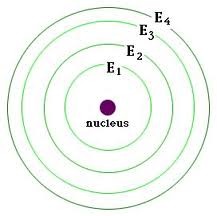
energy level
a region of an atom in which electrons of the same energy are likely to be found
13
New cards
Mixture
A combination of two or more substances that are not chemically combined
14
New cards
homogenous mixture (solution)
a mixture that is the same in its properties throughout given samples

15
New cards
heterogeneous mixture
A mixture that is not the same in composition; components are not evenly distributed throughout the mixture

16
New cards
Solution
What is a mixture where 2 or more substances are uniformly distributed in another substance?
17
New cards
Solute
___________ is the substance dissolved in the solution.
18
New cards
Solvent
___________ is the substance in which the solute is dissolved
19
New cards
Water
What is the universal solvent?
20
New cards
Filtration
A process that separates materials based on the size of their particles.

21
New cards
Sifting
a process used to separate the parts of a mixture by particle size

22
New cards
Periodic Table
an arrangement of elements in which the elements are separated into groups based on a set of repeating properties
23
New cards
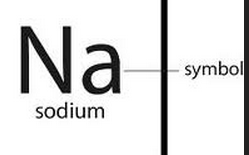
Chemical Symbol
A one or two letter representation of an element
24
New cards
Number of protons
What is the atomic number of an element?
25
New cards
Atomic mass
What do you find by adding the number of protons and neutrons together?
26
New cards
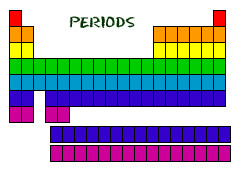
Period
A horizontal row of elements in the periodic table
27
New cards
Group
Vertical column in the periodic table
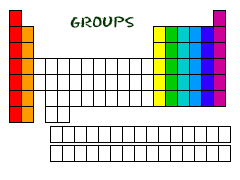
28
New cards
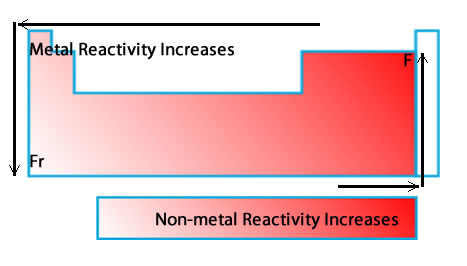
Reactivity
How readily a substance combines chemically with other substances.
29
New cards
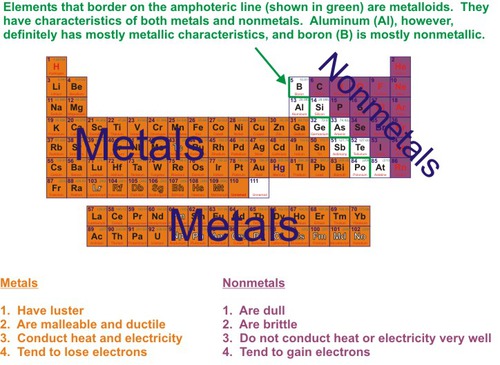
Metals
Elements that are good conductors of electric current and heat.
30
New cards
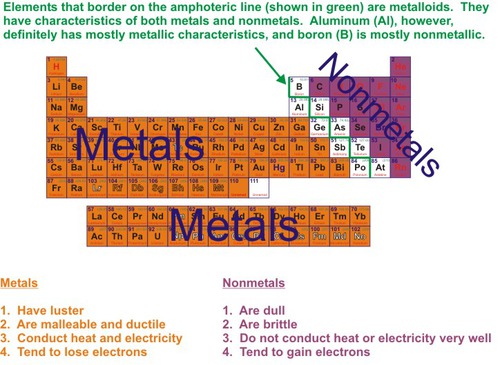
Non-Metals
Low conductivity, not ductile, not malleable, brittle, dull, gas at room temp
31
New cards
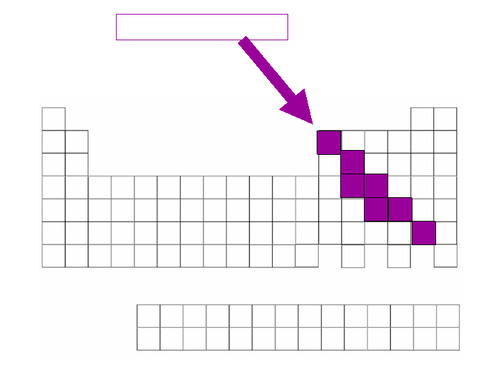
Mettalloids
elements with some of the properties of metals and some of the properties of non-metals. Found along the zig zag line
32
New cards
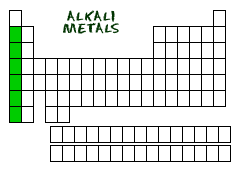
Alkali Metals (Group 1)
Group 1 on the periodic table.
1) Soft metals
2) Reacts quickly with oxygen
3) Reacts violently with water
4) Most reactive group on Periodic Table
1) Soft metals
2) Reacts quickly with oxygen
3) Reacts violently with water
4) Most reactive group on Periodic Table
33
New cards
Alkaline Earth Metals (Group 2)
metallic elements in group 2 of the periodic table which are harder than the group 1 alkali metals but also less reactive than
34
New cards
Transition Metals (Groups 3-12)
Good thermal conductors, shiny, electric conductors, higher density and melting points than group 1+2, 1-2 valence electrons
35
New cards
Halogens (Group 17)
Very reactive non-metals. Atoms of this group have seven electrons in their outer level. Also known for forming salts.
36
New cards
Noble Gases (Group 18)
Elements in group 18 that have a full 8 electrons in the outer energy shell and, therefore, are stable and tend not to form chemical bonds. Least reactive elements on Periodic Table
37
New cards
Chemical Formula
A combination of chemical symbols and numbers to represent a substance

38
New cards
Subscript
A number in a chemical formula that tells the number of atoms in a molecule or the ratio of elements in a compound
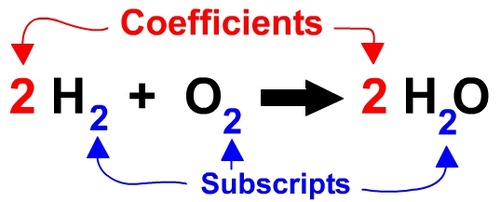
39
New cards
Coefficient
A number in front of a chemical formula in an equation that indicates how many molecules or atoms of each reactant and product are involved in a reaction.
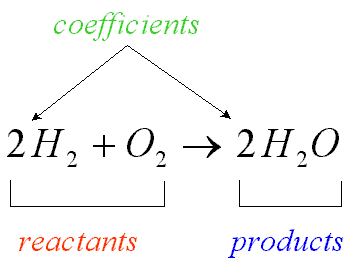
40
New cards
The Yield of a Chemical Reaction is how much you actually made
41
New cards
Chemical Property
A characteristic of a pure substance that describes its ability to change into different substances
42
New cards
Physical Property
A characteristic of a pure substance that can be observed without changing it into another substance

43
New cards
Chemical Change
A change in matter that produces one or more new substances

44
New cards
Physical Change
A change in a substance that does not involve a change in the identity of the substance
45
New cards
catalyst
substance that speeds up the rate of a chemical reaction
46
New cards
Reactant
A chemical substance that is present at the start of a chemical reaction
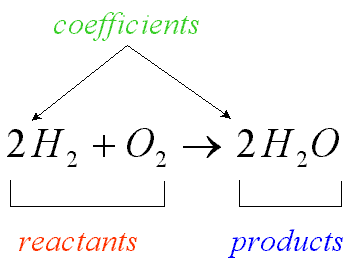
47
New cards
Product
A substance produced in a chemical reaction
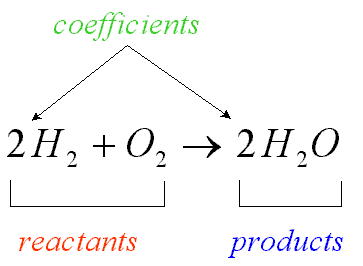
48
New cards
Concentration
A measurement of how much solute exists within a certain volume of solvent
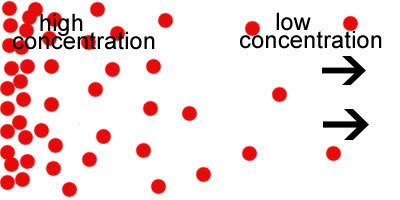
49
New cards
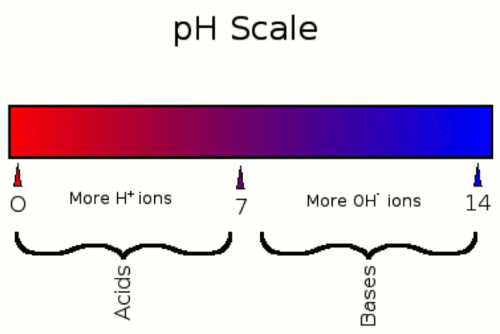
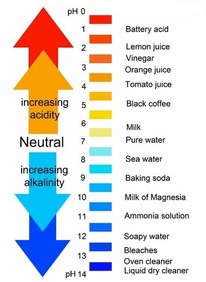
pH Scale
scale with values from 0 to 14, used to measure the concentration of H+ ions in a solution; a pH of 0 to 7 is acidic, a pH of 7 is neutral, and a pH of 7 to 14 is basic

50
New cards
pH Value of &
Substance that is neutral, it's not an acid or base
51
New cards
0-6 Acid
Having a pH less than 7

52
New cards
8-14 Base (Alkaline)
Having a pH greater than 7
53
New cards
combustible
capable of catching fire or burning
54
New cards
malleable
easy to shape or bend

55
New cards
ductile
A term used to describe a material that can be pulled out into a long wire.
56
New cards
Oxidation
A chemical change in which a substance combines with oxygen, as when iron oxidizes, forming rust
57
New cards
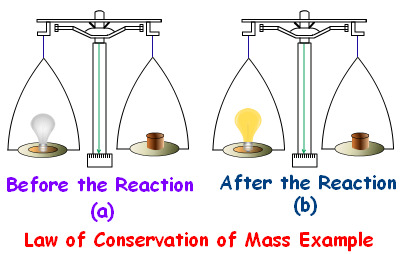
Law of Conservation
matter cannot be created or destroyed, it is all still present.
58
New cards
balanced equation
a chemical equation in which mass is conserved; each side of the equation has the same number of atoms of each element
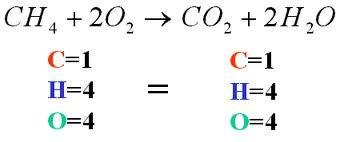
59
New cards
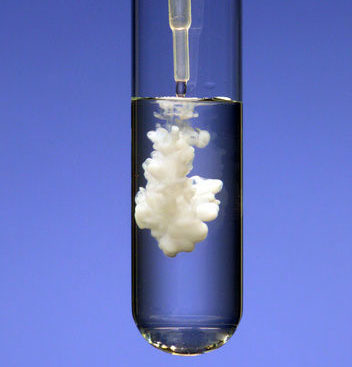
precipitate
A solid that forms from a solution during a chemical reaction.
60
New cards
oxygen, carbon, hydrogen, and nitrogen
What 4 elements make up 90% of an organisms mass?
61
New cards
Isotope
What is it called when an element has a different number of neutrons than normal?