chemistry - electrolysis + half equations
0.0(0)
Card Sorting
1/26
Last updated 4:59 PM on 11/15/22
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
27 Terms
1
New cards
electricity
what type of energy source does electrolysis use?
2
New cards
Al
The half equation for the reaction that happens during aluminium manufacture is shown below, what symbol completes it?
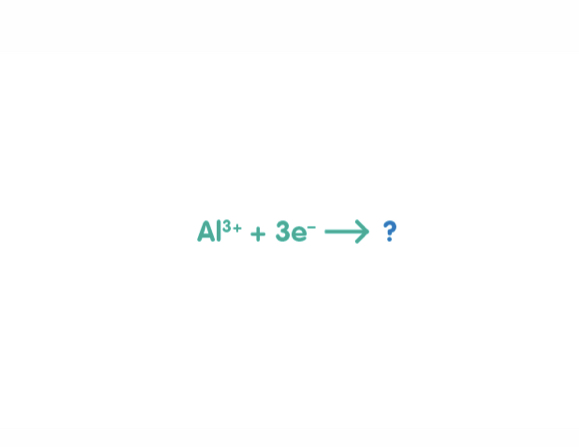
3
New cards
2
What should be the missing number be to balance this half equation?
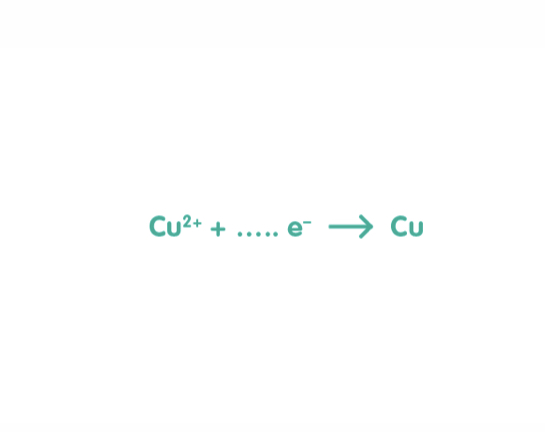
4
New cards
cathode
at which electrode will Copper 2+ gain 2 electrons and become Copper?
5
New cards
reduced, cathode
positive ions are _______ (gain) at the ________ (electrode)
6
New cards
electrolysis
what process is usually used to extract metals that are more reactive that carbon
7
New cards
electrolysis
the process in which an electric current is used to produce a chemical reaction, such as the decomposition of water
8
New cards
cryolite
what is aluminum oxide often mixed with to reduce the melting point
9
New cards
aluminum metal
what is extracted from aluminum oxide by electrolysis
10
New cards
graphite
what are positive electrons made of
11
New cards
negative oxide ions
which ions in aluminum oxide will be attracted to the positive electrodes
12
New cards
cathode
metal ions are attracted to which electrode during electrolysis?
13
New cards
cathode
negative electrode at which reduction occurs
14
New cards
anode
positive electrode at which oxidation occurs
15
New cards
positive
what ion does hydrogen form
16
New cards
cathode
hydrogen ions are attracted to which electrode during electrolysis?
17
New cards
sodium
during the electrolysis of molten sodium chloride, which ion discharges at the cathode
18
New cards
chlorine
during the electrolysis, of molten sodium chloride which Ion discharges at the anode
19
New cards
aluminum oxide
what is the name of this important compound found in aluminum ore

20
New cards
reacts with oxygen and corrodes
why should the carbon anode be replaced regularly?
21
New cards
reduced
positive metal ions are
22
New cards
oxidised
negative non metal ions are
23
New cards
uncharged, discharged
as ions gain or lose electrons they form the ______ element and are ________ from the electrolyte
24
New cards
1+ ions
group 1 alkali metals form
25
New cards
2+ ions
group 2 metals form
26
New cards
-2 ions
group 6 transition metals from
27
New cards
-1 ions
group 7 halogens form