General chemistry 11: oxidation-reduction reactions
1/13
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
14 Terms
Any free element or diatomic species has an oxidation number of [#]
0

A monatomic ion has an oxidation charge that equals [...]
the charge of the ion
When in a compound, metals have the following oxidation states:
Group 1A metals = [#]
Group 2A metals = [#]
+1
+2
When in a compound, group 7A metals have an oxidation state of [#] unless combined with an element of greater electronegativity
-1
The oxidation state of hydrogen in a compound is [#], except for metal hydrides, such as NaH, LiH, etc., in which the oxidation state for H is[...]
+1
-1

The oxidation state of oxygen in a compound is [#],except for peroxides like H2O2, and Na2O2, in which the oxidation state for O is[#]
-2
-1
The sum of the oxidation numbers in a neutral compound is [#]
0

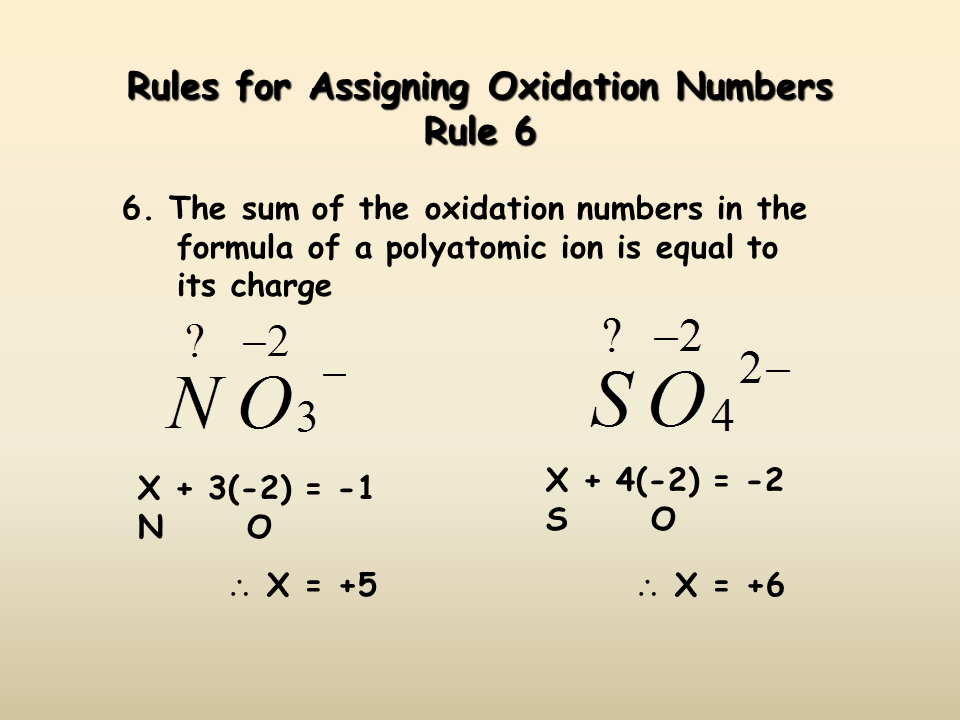
The sum of the oxidation numbers in a polyatomic ion is equal to [...]
the charge of the ion
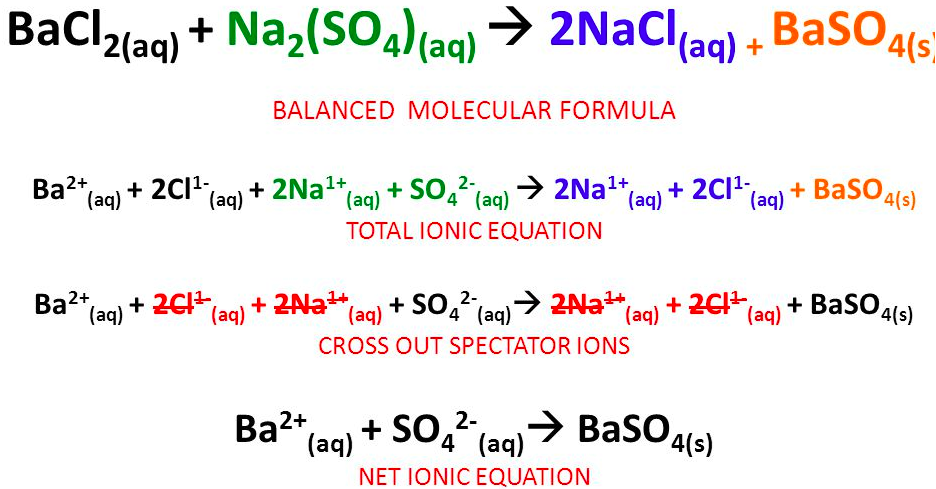
The [... equation] shows all of the ions present in a reaction including spectator ions
complete ionic equation
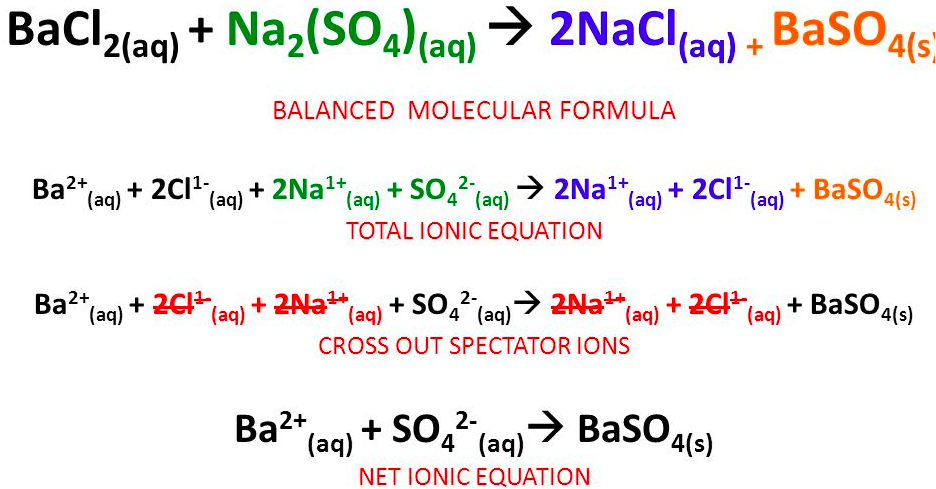
The [... equation] is a chemical equation for a reaction which lists only those species participating in the reaction
net ionic equation
does not include spectator ions
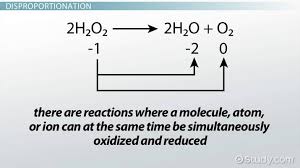
[...], also known as [...], is a REDOX reaction where a molecule is transformed into two or more dissimilar products
disproportionation also known as dismutation
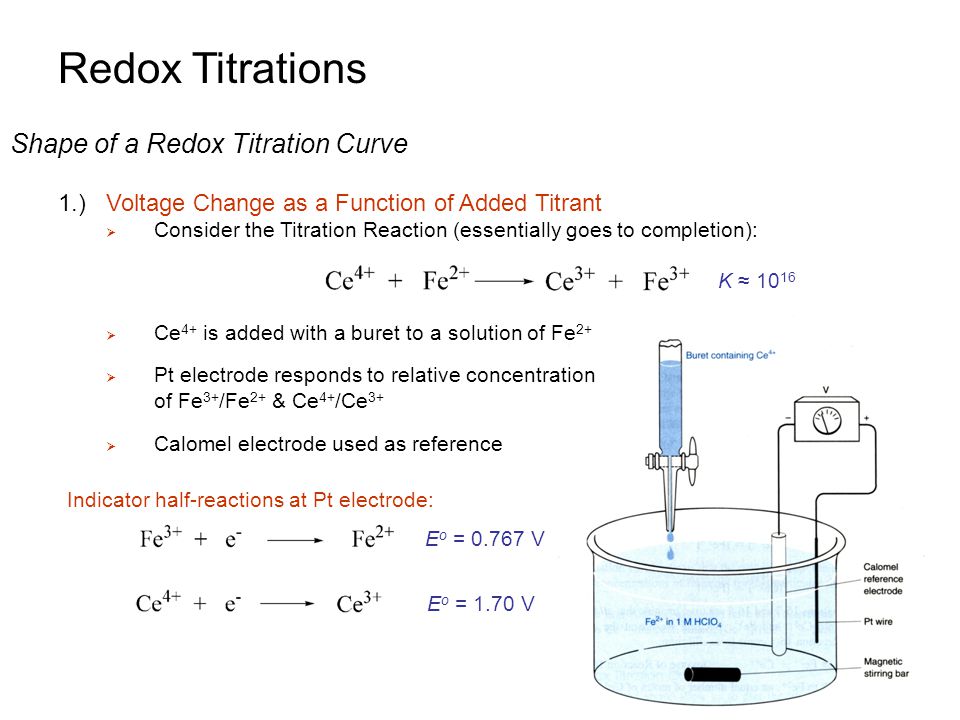
A/an [... titration] is similar in methodology to acid-base titrations, however, these titrations follow transfer of charge
redox titration
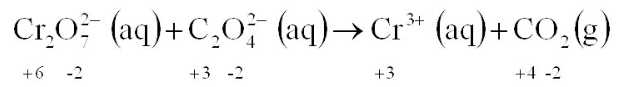
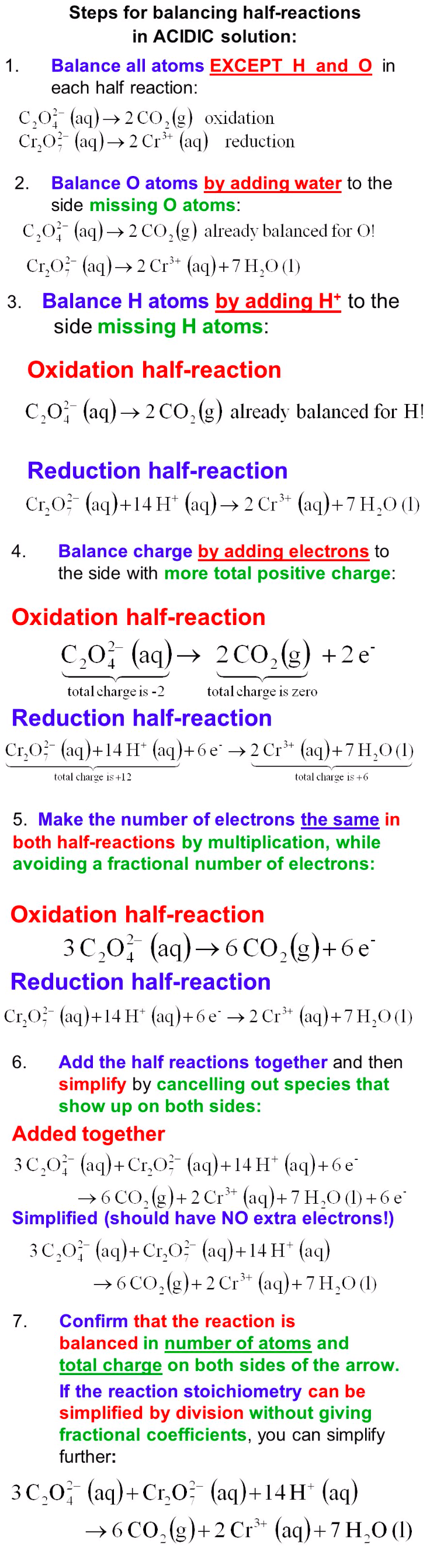
Balance the above REDOX reaction in an acidic solution:
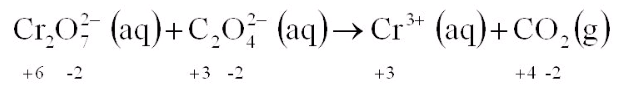
Balance the above REDOX reaction in a basic solution:
