enthalpy: direct methods
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
what is an enthalpy change?
the heat change of a system measured at constant pressure
what are the standard conditions for enthalpy measurements?
100kPa or 1atm. pressure and a temperature of 298 K (25°C)
what can standard conditions also mean?
the reactant and products must be in physical states i.e. solid, liquid, gas, that are normal under these conditions i.e. their standard states
any solutions have a concentration of 1 mol/dm³.
equation for enthalpy change
Delta H = H{products} - H{reactants}
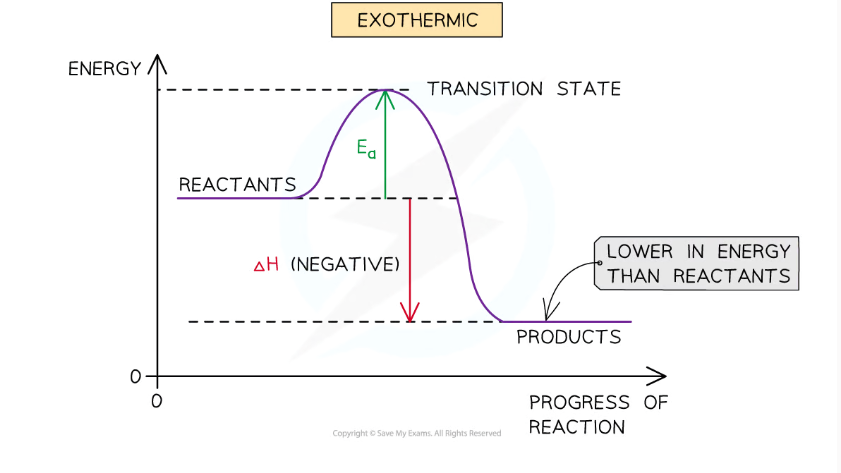
exothermic conditions:
energy is transferred, chemical system loses energy and the surroundings gain energy and increase in temperature. This shows that the value of delta H is negative.
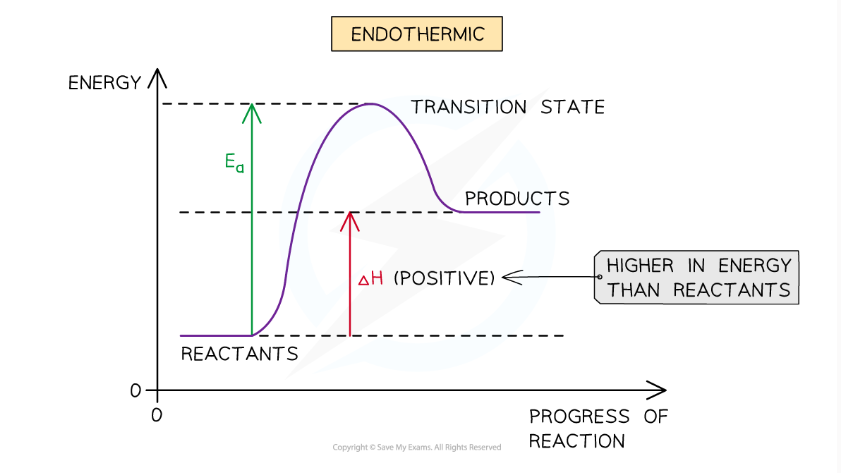
endothermic reactions:
chemical system that absorb/gains energy from the surroundings, resulting in a decrease in temperature and surroundings losing enegy. This indicates that the value of delta H is positive.
what is activation energy?
chemical reactions that have an energy barrier that prevents the reaction from occurring until this energy is overcome. It is the minimum amount of energy required to start a reaction by breaking of bonds.
why is Ea important?
exothermic reactions would occur spontaneously and fuels would spontaneously combust.
how can you calculate Ea of a reaction using a diagram?
the difference between the energy of the activated complex and the energy of the reactants.
what is standard enthalpy change of formation? (delta fH^o)
the enthalpy change that occurs when 1 mole of a compound in its standard state is formed from its constituent elements in their standard states under standard conditions.
e.g. C (s) + 2H2 (g)→CH4 (g)
what is standard enthalpy change of combustion? (delta cH^o)
the enthalpy change when one mole of a substance is completely burned in oxygen under standard conditions, resulting in the formation of products in their standard states.
e.g. CH4 (g) + 2O2 (g)→ CO2 (g) + 2H2O (L) (always delta negative since combustion is exothermic)
what is standard enthalpy change of reaction? (delta rH^o)
the enthalpy change that occurs when a reaction takes place under standard conditions, resulting in products in their standard states.
what is standard enthalpy change of neutralisation? (delta nH^o)
the enthalpy change that occurs when one equivalent of an acid reacts with one equivalent of a base to form one mole of water and salt under standard conditions.
e.g. HCL (aq) + KOH (aq) → KCl (aq) + H2O (l)
ways to determine enthalpy change
calorimetry, Hess's law, and standard enthalpy values.
what is calorimetry?
measurement enthalpy changes in chemical reactions, typically using a calorimeter which can also be made using a polystyrene cup, vacuum flask, or metal can.
how to use q=mc^T
q = energy change, m = mass of substance heated e.g. water, c = 4.18, ^T = change in temp. then to work out enthalpy change you divide this answer by the moles of substance used.
how to use a calorimeter?
make sure you use a heat insulated vessel and use known amounts of all reactants and known volumes of liquids - one reactant should be in excess, to ensure a complete reaction occurs, then measure temperature changes to calculate enthalpy.
3 bullet ways that a bomb calorimeter is an improvement over the calorimeter used in labs
more accurate measurements of heat changes because it is a constant volume system,
minimizes heat loss,
and allows for the measurement of high-temperature reactions, which improves precision in calculating enthalpy changes.
how does calculating enthalpy of neutralisation differ?
the m = for both acid + base
what’s a cooling curve?
A graph that shows the change in temperature of a substance as it cools, illustrating phase transitions such as freezing and solidification, makes it easier to estimate the point of neutralisation or to gain an accurate temperature change for enthalpy calculations.
why is the cooling curve method more accurate?
it accounts for the heat gained or lost.
what is enthalpy?
all heat energy present in a chemical system (chemicals present in a reaction).
why will a published value be higher than the experimetnal value?
Leaving cap off the unlit spirit burner will evaporate some fuel, making it seem like more fuel was burned.
2. Incomplete combustion may also occur, resulting in additional heat loss and affecting the measured temperature change.
3. Alot of heat energy released was lost to surroundings, instead of water it went to air or metal calorimeter.
4. may not have been carried out under standard conditions.
how to calculate standard enthalpy change of reaction for a displacement reaction
mg + cuso4 →mgso4 + cu
add mg powder to 100cm of a conc of 1moldm-3 (1mol) of cuso4 solution, cuso4 should be in excess so put less mg, 0.05mol mg (1.22g), put soltuion in polysyrene cup to redce heat loss to the environment, put in glass beaker to prevent tipping.
make sure this is done at room temp by taking temp readings every 30s until constant reached, continue taking readings every 30s for 5 mins.
Measure temperature change and use q = mcΔT to calculate the enthalpy change. (use stiochiometry for the amount of reactants to find standard enthalpy change per mole.
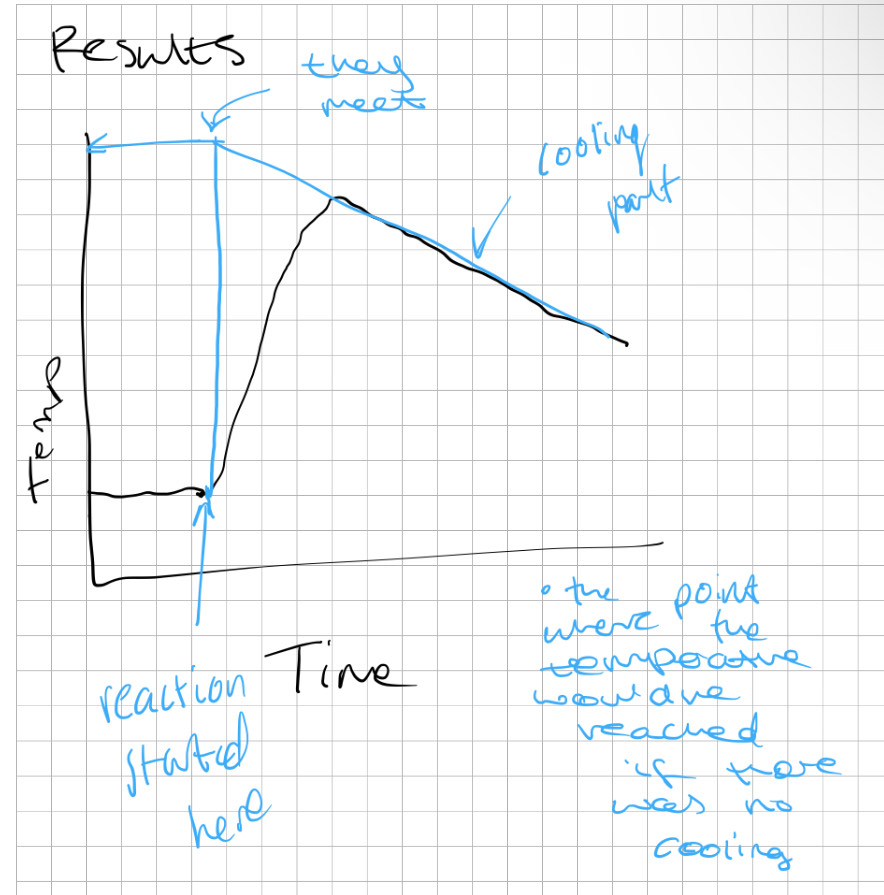
why is a cooling curve needed?
to measure final temp change accurately, since the temp increases and decreases due to thermal energy being lost to surroundings e.g. air.