IMED1003 - Buffers + Acid Base Balance (L24)
1/31
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
32 Terms
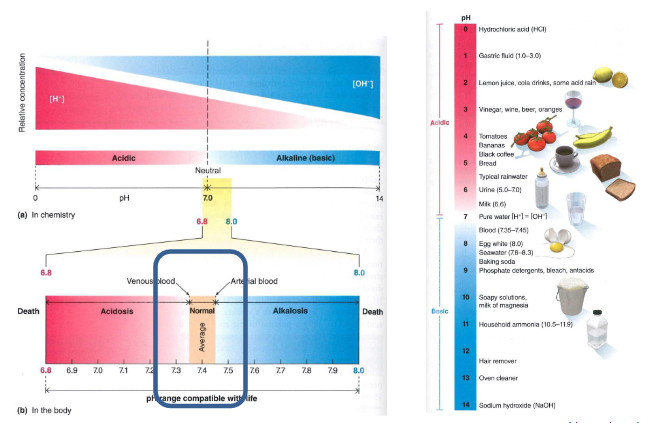
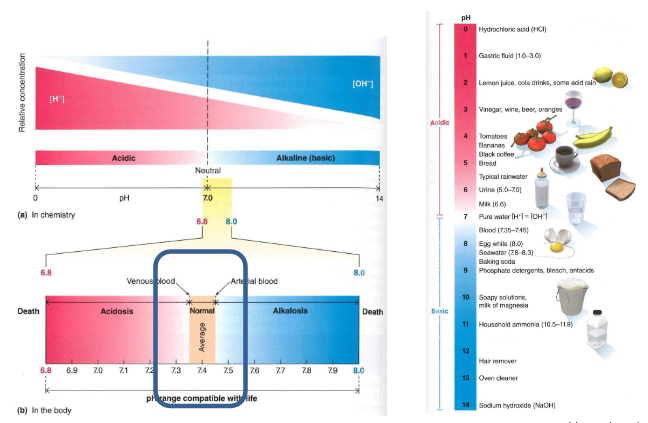
pH chemistry and in the body
DIAGRAM ON SLIDE 4
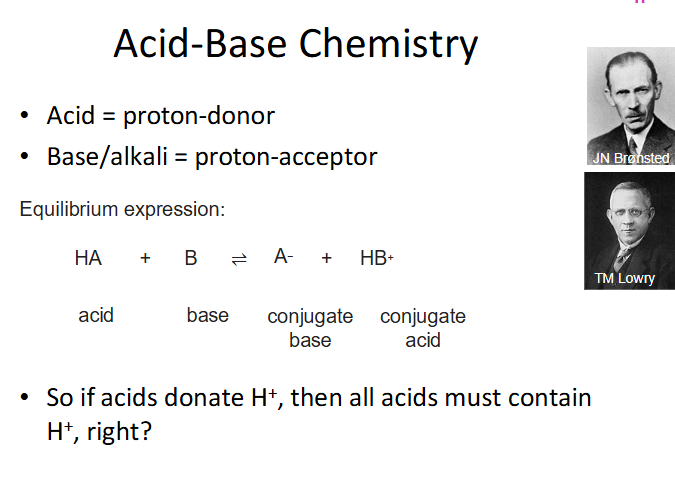
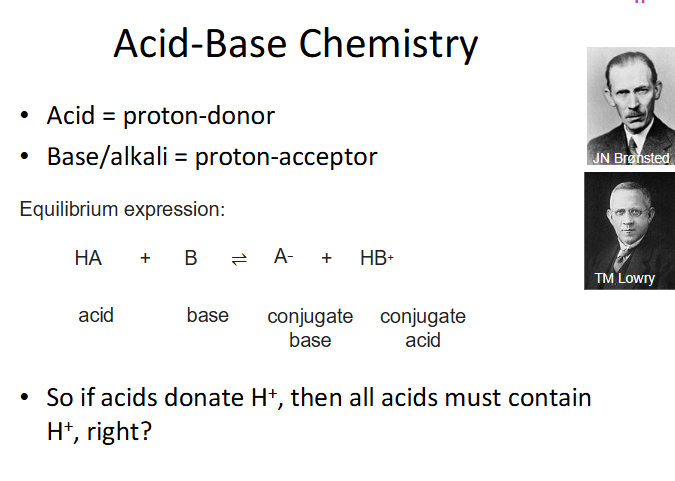
Acid Base Chemistry
DIAGRAM ON SLIDE 5 (WHOLE SLIDE)
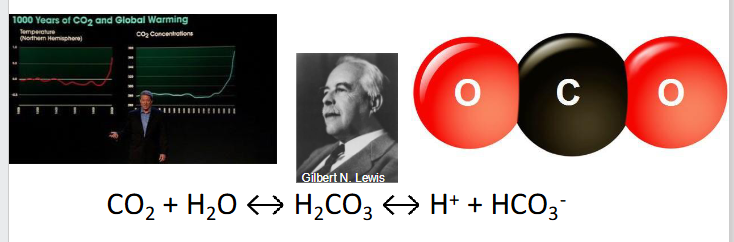
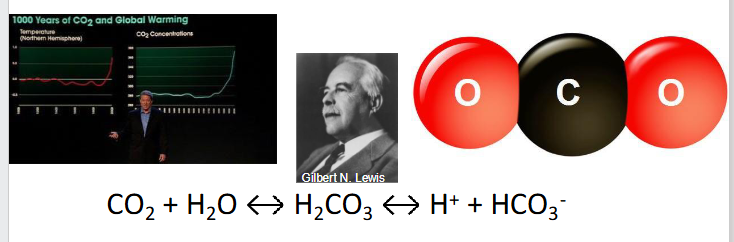
The most important acid to human physiological homeostasis is CO2
- CO2 + H2O <--> H2CO3 <-> H+ + HCO3-
- CO2 is a lewis acid (accepts electron pair from H2O, liberating H+)
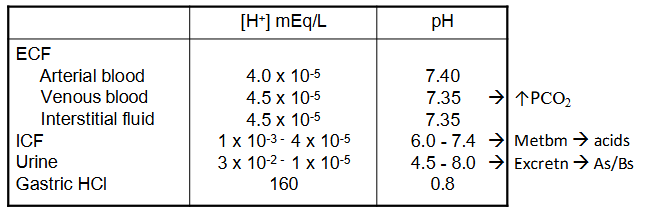
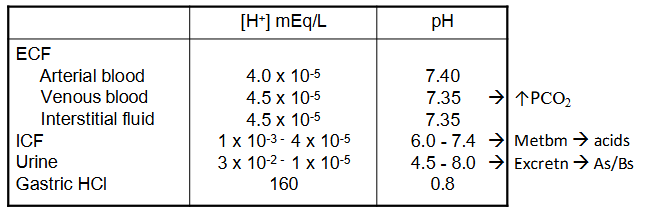
Acid-Base Homeostasis
- ECF [H+] = 0.00004 mEq/L = 40 nEq/L
- normal [H+] variation = plus minus 3-5 nEq/L (probs wont die unless <10 or >160)
.
- pH = -log[H+]
- pH = -log (0.00000004) = 7.4
.
mEq/L means milli equivalents per L, aka millimoles per litre
Acidosis (process), Acidaemia (state)
- increase in acidity ([H+]) of body fluids
- Reduction in arterial pH <7.35
![<p>- increase in acidity ([H+]) of body fluids</p><p>- Reduction in arterial pH <7.35</p>](https://knowt-user-attachments.s3.amazonaws.com/0677d3a3-b58a-415e-8c8a-b653379a7aae.png)
Alkalosis (process), Alkalaemia (state)
- reduction in acidity ([H+]) of body fluids
- increase in arterial pH >7.45
![<p>- reduction in acidity ([H+]) of body fluids</p><p>- increase in arterial pH >7.45</p>](https://knowt-user-attachments.s3.amazonaws.com/b880920c-8541-41a7-81db-5eb76123cd27.png)
-aemia
blood
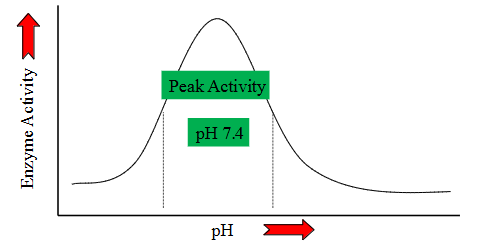
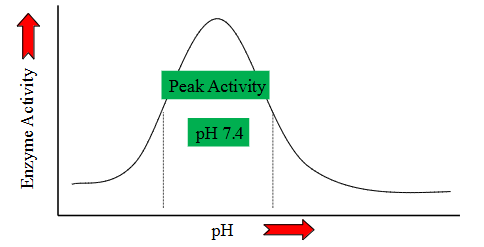
pH effects on Metabolism
- practically every step of every metabolic process is pH dependent
- Deviate from optimal pH - decreased reaction efficiency
- this is because of enzymes
pH effects on neuromuscular system
- acidosis -> inhibitory (inhibits signalling)
- alkalosis -> excitatory (too much excitation of signalling)
.
WHY?
Acidosis increases free [Ca2+]
- Ca2+ binding to albumin is pH dependent
- Ca2+ blocks vNa+ channels -> raises AP threshold
.
K+ Balance:
- Acidosis -> increase in serum [K+]
- Alkalosis -> decrease in serum [K+]
Consequences of Acidosis
- headaches, confusion, lethargy, tremors, sleepiness
- Cerebral dysfunction -> coma
- Cardiovascular dysfunction
- hyperventilation
Consequences of Alkalosis
- muscular weakness, pain, cramps, spasms (smooth and skeletal muscle) -> tetany
- hypoventilation
Consequences
IMPACT DEPENDENT ON SEVERITY OF ILLNESS
- Anaesthesia
- Intensive care (exercise)
- Emergency medicine
- Respiratory medicine
- Nephrology
Normal physiology promotes acidosis
- whie living, eating and drinking there is production of 1mmol of acid/kg body weight per day (70kg = 70mmol/day)
- most acid comes from CHO/fat metabolism (generates 15000 to 20000 mmol of CO2 daily
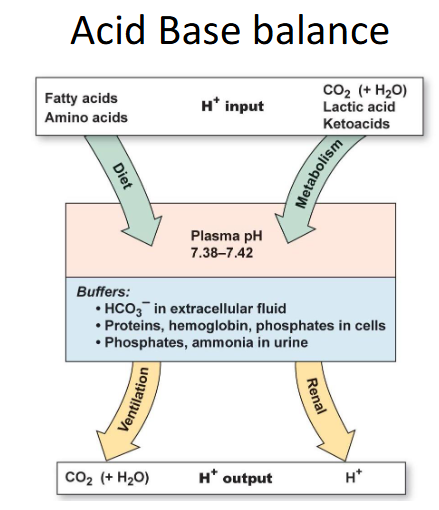
Acid Base Balance
- we can either breathe out CO2 or excrete it in the urine to get rid of acids
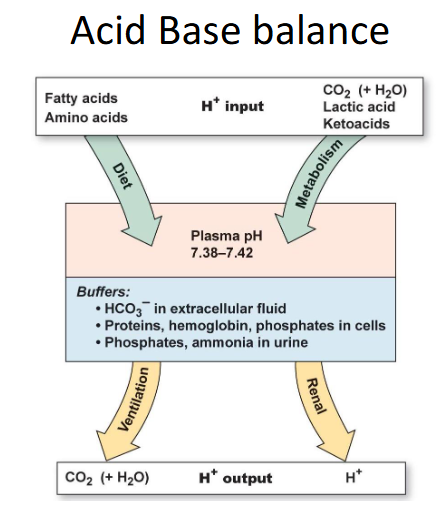
Defense Mechanisms
CHEMICAL BUFFERING (IMMEDIATE - BUT EXHAUSTIBLE)
- Solutions that resist changes in pH
- intracellular and extracellular buffers provide an immediate response to acid-base disturbances (bone also buffers acid loads)
- they can't get rid of acid from the body, they can only temporarily reduce its impact short term
.
Pulmonary regulation (minutes-hours - limited question)
- [CO2] is regulated by changes in breathing frequency and depth
- as CO2 is exhaled -> blood pH increases
.
RENAL REGULATION (HOURS-DAYS, VERY POWERFUL, INFINITE CAPACITY):
- Kidneys control adjust the amount of HCO3- and/or H+ that is excreted
- Excreting HCO3- -> decrease blood pH, excreting H+ -> increase blood pH
Buffer Systems
- Buffer = substance that reversibly consumes or releases H+ to decrease changes in pH
- made up of a weak acid and its conjugate base (conjugate base can accept H+ and the weak acid can donate H+ -> minimising changes in free [H+]
- Buffer- + H+ <--> H-Buffer
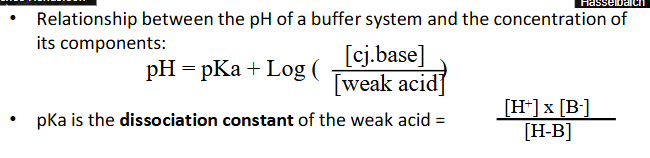
Henderson-Hasselbach equation
- Relationship between the pH of a buffer system and the concentration of its components:
- pH = pKa + log (whatever on diagram)
- pKa is the dissociation constant of a weak acid
- So ... pKa determines optimal pH for max. buffering capacity (a buffer system works best to minimise pH changes near its pKa)
- and the relative [cj.base]:[acid] determine how much acid or base can be buffered -> i.e acid-buffering capacity, base-buffering capacity
![<p>- Relationship between the pH of a buffer system and the concentration of its components:</p><p>- pH = pKa + log (whatever on diagram)</p><p>- pKa is the dissociation constant of a weak acid</p><p>- So ... pKa determines optimal pH for max. buffering capacity (a buffer system works best to minimise pH changes near its pKa)</p><p>- and the relative [cj.base]:[acid] determine how much acid or base can be buffered -> i.e acid-buffering capacity, base-buffering capacity</p>](https://knowt-user-attachments.s3.amazonaws.com/9cfdf9af-da64-4892-8561-6453604d6f15.png)
Buffer Power
- buffer power is determined by the "pH appropriateness" of the system (does pKa match pH?)
- capacity determined by: total [buffer] and relative [H-B] and [B-]
![<p>- buffer power is determined by the "pH appropriateness" of the system (does pKa match pH?)</p><p>- capacity determined by: total [buffer] and relative [H-B] and [B-]</p>](https://knowt-user-attachments.s3.amazonaws.com/f302c46b-54bb-432b-9700-c98fdedb45ec.png)
Phsyiologically-Relevant Buffer Systems
- there are certain physiologicla situations where we need different buffers
.
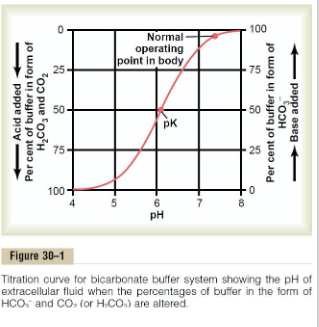
BICARBONATE: HCO3- (pKa = 6.37)
- Dominant ECF buffer
.
AMMONIA: NH3 (pKa = 9.25)
- ECF too, but mostly in renal tubules
.
PHOSPHATE: HPO4 2- (pKa = 7.21)
- ICF buffer
.
PROTEINS (many close to pKa of around 7.4, Hb around 6.8)
- ICF buffer (ie - Hb in RBCs), 60-70% of total buffering capacity
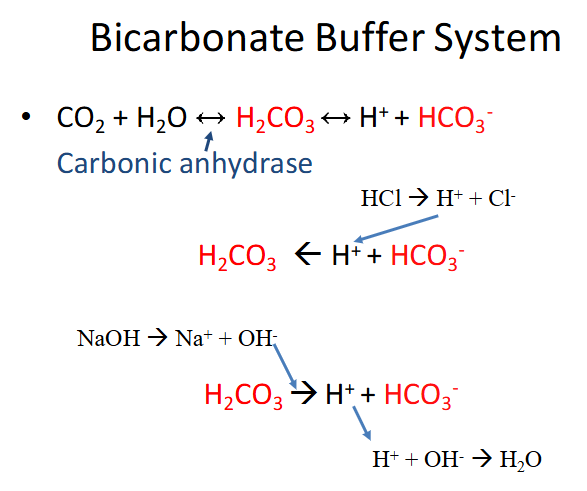
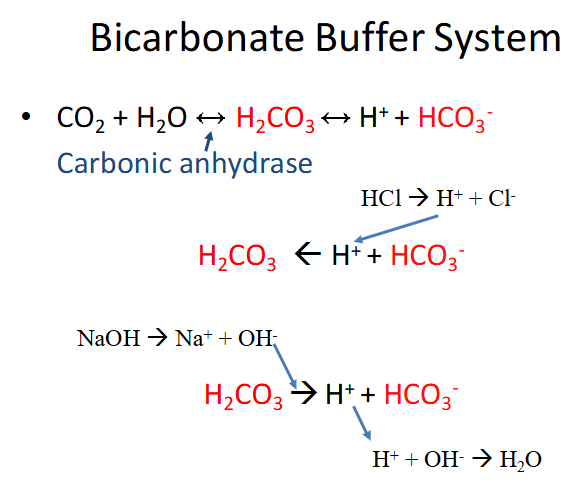
Bicarbonate Buffer System
- carbonic anhydrase is the enzyme
- bottom parts shows it being driven in both directions (buffering against acid and buffering against base)
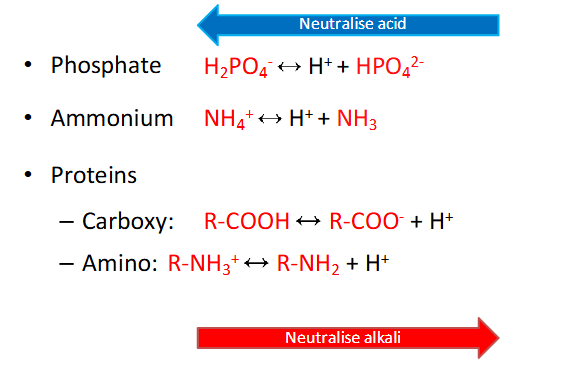
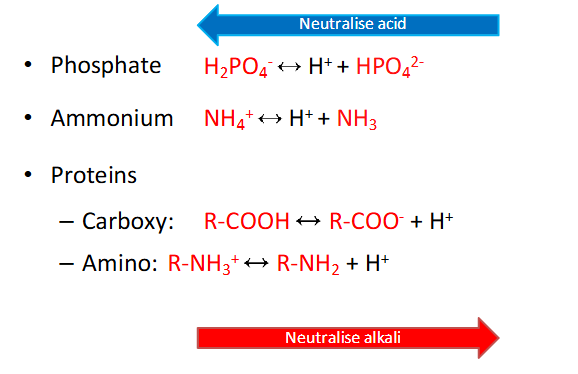
The Others
- dont need to memorise these equations (probably have to considering IMED)
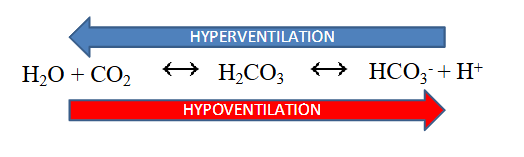
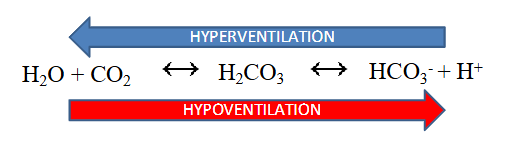
Respiratory Control
- ventilation rates control pH balance (increased ventilation removes CO2)
- hyperventilation drives reaction to the left (decreased H+, increaesd pH)
- hypoventilation drives reaction to the right (increased H+, decreased pH)
.
- we would call abnormal loss of CO2 respiratory alkalosis since its coming form respiratory and its alkalopsis
- respiratory acidosis is more profound then alkalosis since there is a stimulus to breathe when CO2 is high but no stimulus when its low
Effect of ventilation on pH
- increase Va x2 --> increased pH by 0.23 (7.4 -> 7.63)
- decreased Va to 1/4 --> decreased pH by 0.45 (7.4 -> 6.95)
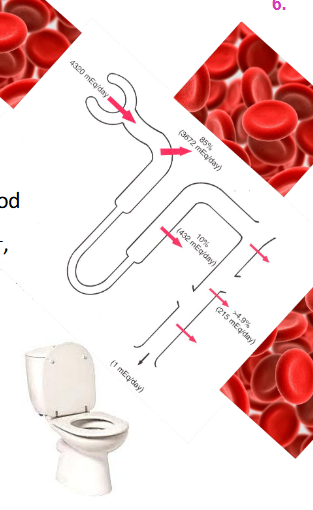
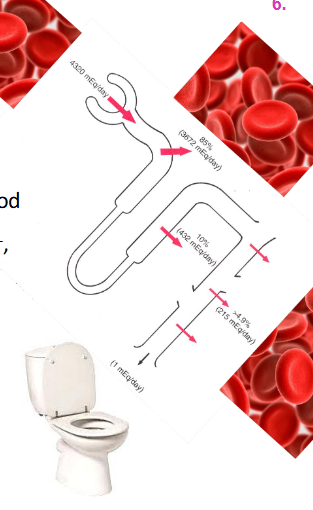
Renal Control
- kidneys constantly remove (filter) HCO3- (base) from blood
- to maintain balance, we must reabsorb enough HCO3- back into blood
- BUT kidneys cannot reabsorb HCO3- it must first b e converted:
HCO3- + H+ <--> H2CO3 <--> H2O + CO2
- To do this, kidneys must secrete H+ (1:1 ratio with HCO3-)
- Quantitative H+ secretion determines HCO3- reabsorption
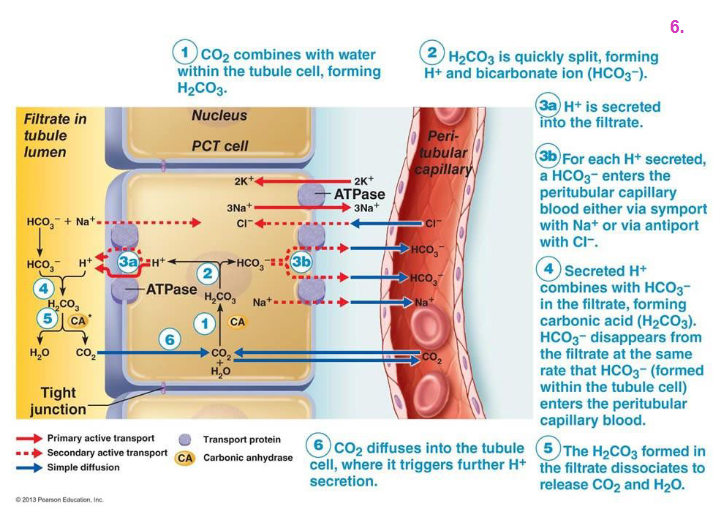
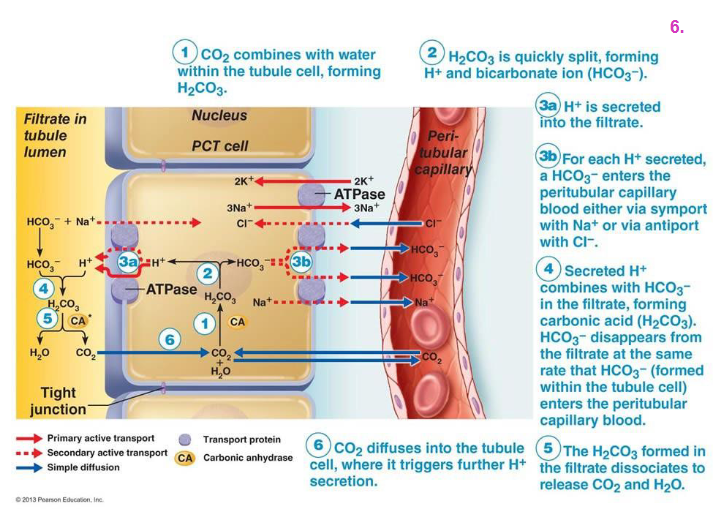
Renal Control EXPLAINED DIAGRAM
- basically the kidney cell uses carbonic anhydrase to make HCO3- and H+.
- the bicarbonate goes back into the blood, the H+ is excreted
Renal Control and H+ secretion
- Result: excretion of acidic or basic urine
- Balance between: H+ secretion / HCO3- filtration
- H+ secretion = HCO3- filtration = no change
- H+ secretion > HCO3- filtration = acid loss
- H+ secretion < HCO3- filtration = base loss
Respiratory and Metabolic Control
- Respiratory control changes blood [CO2] (PCO2)
- Metabolic control (renal) changes blood [HCO3-]
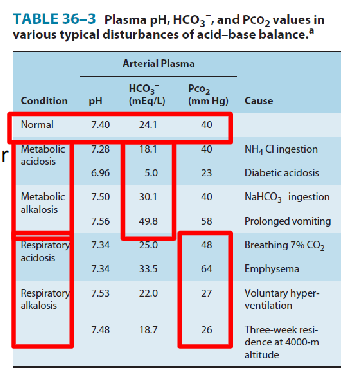
Acid Base Disorders
Cause - metabolic or respiratory? (we can determine whether or not its metabolic or respiratory by looking at the following factors):
.
METABOLIC:
- due to production, ingestion or loss of acids/bases
- change plasma [HCO3-]
.
RESPIRATORY:
- due to hyper/hypoventilation
- change in PCO2 (ventilation)
.
- basically we can determine the source of error. is the kidney having trouble excreting?
- it can be metabolic or respiratory acidosis or alkalosis
![<p>Cause - metabolic or respiratory? (we can determine whether or not its metabolic or respiratory by looking at the following factors):</p><p>.</p><p>METABOLIC:</p><p>- due to production, ingestion or loss of acids/bases</p><p>- change plasma [HCO3-]</p><p>.</p><p>RESPIRATORY:</p><p>- due to hyper/hypoventilation</p><p>- change in PCO2 (ventilation)</p><p>.</p><p>- basically we can determine the source of error. is the kidney having trouble excreting?</p><p>- it can be metabolic or respiratory acidosis or alkalosis</p>](https://knowt-user-attachments.s3.amazonaws.com/e5ab70f5-13cd-44c8-941a-2f3d0f85df62.png)
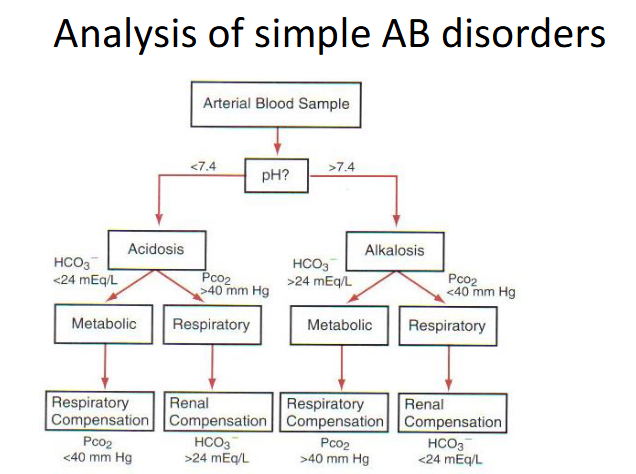
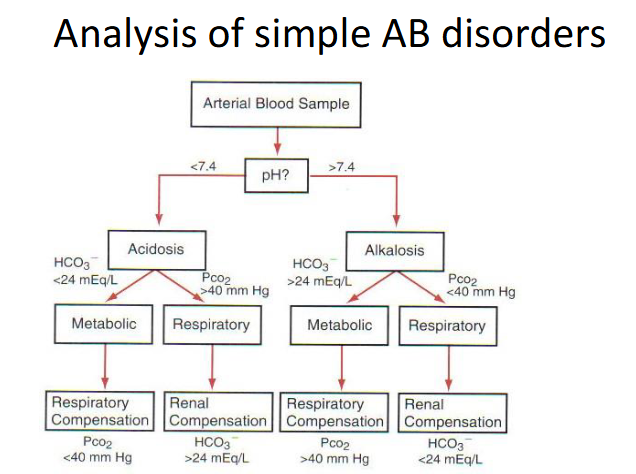
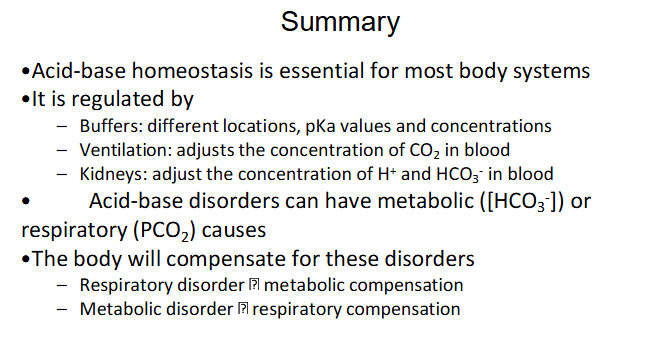
Homeostatic Compensation
RESPIRATORY DISORDER -> Metabolic Compensation
- Primary change in PCO2 -> change in pH -> change in HCO3-
.
METABOLIC DISORDER -> Respiratory Compensation
- Primary change in HCO3- -> change in pH -> change in PCO2
.
METABOLIC COMPENSATION (KIDNEYS):
- Acidosis: increased H+ excretion, total HCO3- reabsorption
- Alkalosis: decreased HCO3- reabsorption -> excreted in urine
.
RESPIRATORY COMPENSATION (LUNGS):
- Acidosis: increased ventilation -> decreased PCO2 -> increased pH
- Alkalosis: decreased ventilation -> increased PCO2 -> decreased pH
Simple AB Disorders
DIAGRAM ON SLIDE 31
Summary
DIAGRAM ON SLIDE 33

QUESTION
DIAGRAM ON SLIDE 34
