Synthetic Polymers - IGCSE Edexcel Chemistry
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
20 Terms
Name of polymer for ethene
poly(ethene)
Name of polymer for propene
poly(propene)
Name of polymer for chloroethene
poly(chloroethene)
Name of polymer for tetrafluoroethene
poly(tetrafluoroethene) aka teflon
Common use of poly(ethene)
plastic bags and bottles
Common use of poly(propene)
ropes and crates
Common use of poly(chloroethene)
electrical cable insulation
Common use of poly(tetrafluoroethene)
non-stick coatings on cooking pans
Monomer
A simple compound whose molecules can join together to form polymers
Polymer
A long chain molecule made of monomers joined together
Addition Polymerisation
The process in which unsaturated alkene molecules (monomers) add on to a growing polymer chain one at a time to form a very long saturated molecular chain (the addition polymer).
C=C
what is needed for a monomer to undergo an addition reaction
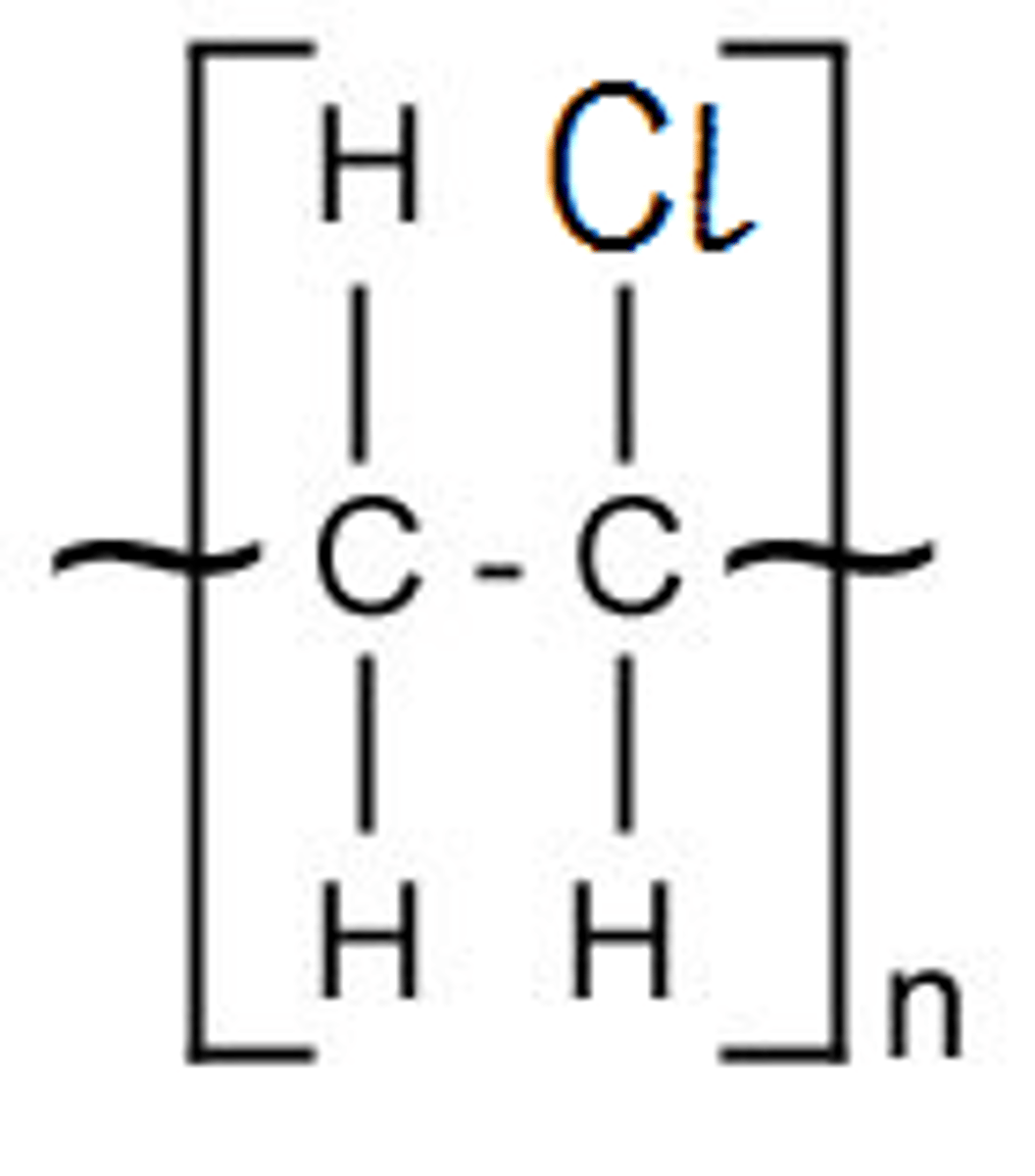
Single repeat unit of poly(chloroethene)
Balanced equation for polymerisation of propene
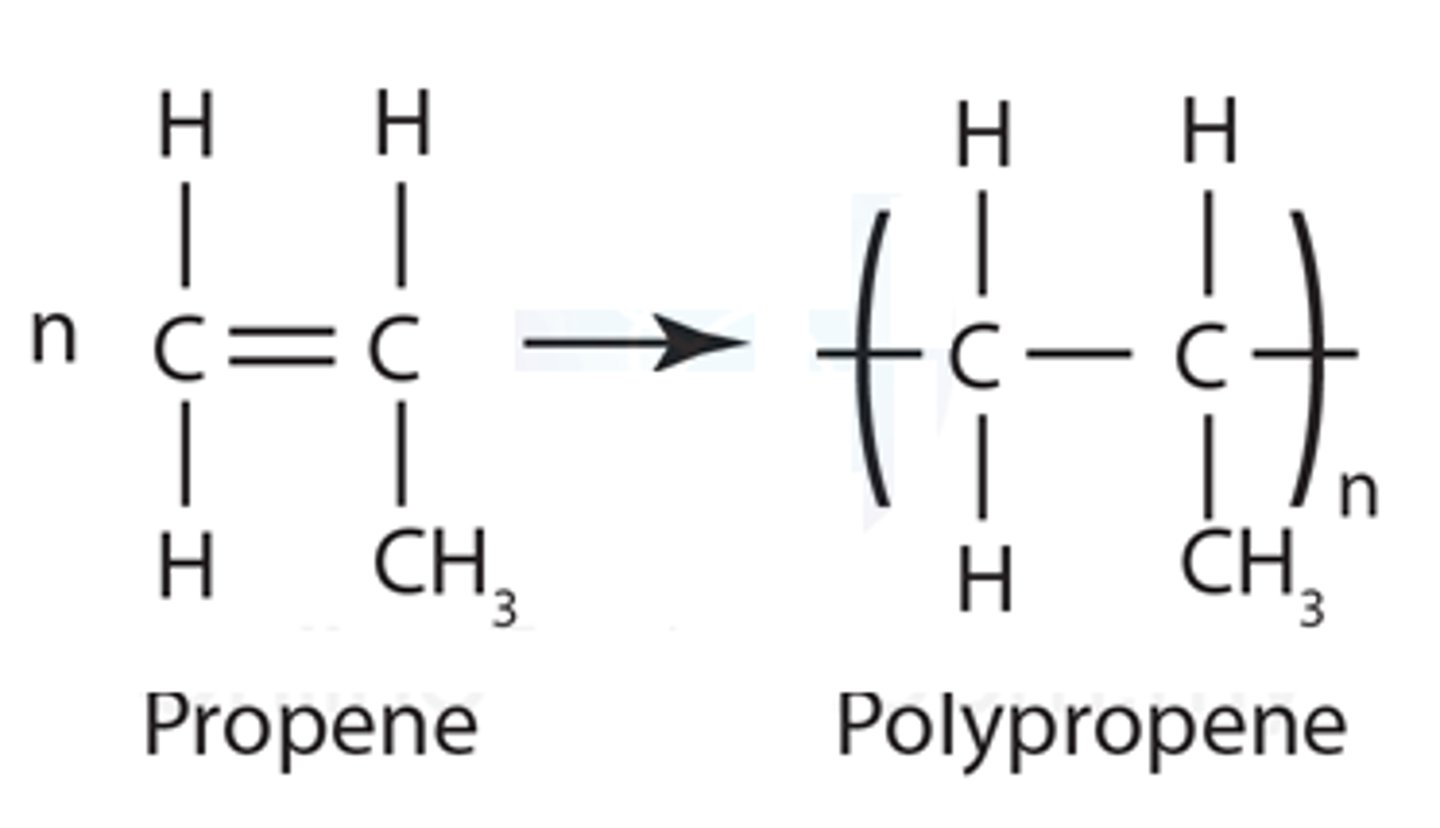
During addition polymerisation......
the C=C breaks and joins up with neighbouring molecules
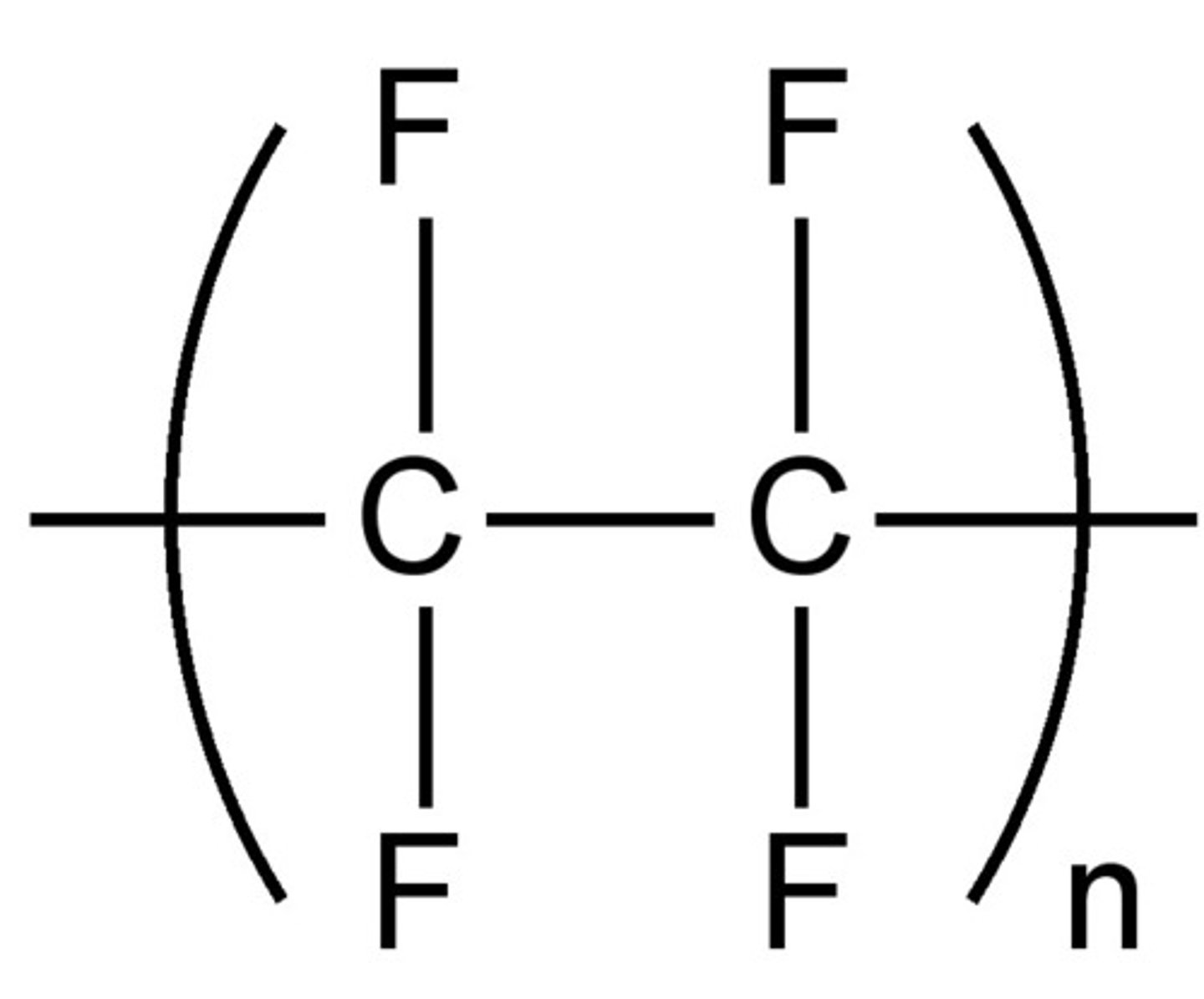
Single repeat unit of poly(tetrafluoroethene)
Non-biodegradable
Cannot be broken down by microorganisms
Condensation Polymerisation happens when......
...... two functional groups react together to form a polymer and water
A polyester is formed when .......
...... a diol reacts with a dicarboxylic acid