2.2.1 Electronic Structure
1/25
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
26 Terms
What's the principal quantum number?
n,
Tells us the shell or energy level that the electrons occupy, and represents the relative overall energy of each orbital in the shell.
Sets of orbitals with the same principal quantum number are refered to as shells or energy levels.
The larger the value of n, the further the shell is from the nucleus and the higher the energy level.
Formula to find how many electrons are in each shell
2n²
n = shell no.
What is the electronic structure of block s elements.
Their highest energy/ outer electron is in an s orbital
How many electrons can occupy the first shell in total?
2 electrons
The first shell has an s sub-shell which contains one orbital which can hold 2 electrons.
How many electrons can occupy the second shell?
8 electrons
Contains a s sub-shell and a p sub-shell
P sub-shells contain 3 orbitals which can hold up to 6 electrons, s sub-shells contain one orbital which can hold up to 2 electrons.
How many electrons can be in the third shell?
18 electrons
The third shell contains a s, p, and d sub-shells
D sub-shells contains 5 orbitals which can hold up to 10 electrons in total, P sub-shells contain 3 orbitals that can hold up to 5 electronsS, S sub-shells contain one orbital which can hold up to two electrons
Definition of a shell
A group of atomic orbitals with the same principal quantum number
Definition of atomic orbitals
A space around the nucleus occupied by up to 2 electrons with opposite spin.
Definition of subshell
A group of orbitals at a given energy level
How many electrons can the fourth shell hold up to?
32 electrons
What's is an s orbital?
Spherical shape
Each shell in an atom contains one s-orbital
What is the shape of a p orbital?
3d dumb-bell shape
What are the rules for filling electron shells?
1) Aufbau principle- the lowest energy sub-levels are occupied first
The 4s sub-shell will be filled before the 3d subshell because it has a slightly lower energy level, this is why when ionising 4s electrons are lost before 3d
2) Hund's rule - single electrons occupy all empty orbitals within a sublevel before they start to form pairs in orbitals.
3) Pauli exclusion principle and spin - each orbital can occupy a maximum of two electrons
4) For CU and Cr 4s electrons are promoted to 3d electrons to half fill or completely fill the 3d sub-shell which makes the atom more stable
What's in a p sub-shell?
Contains three p-orbitals at right angles to each other
Which means 6 electrons can occupy a p subshell
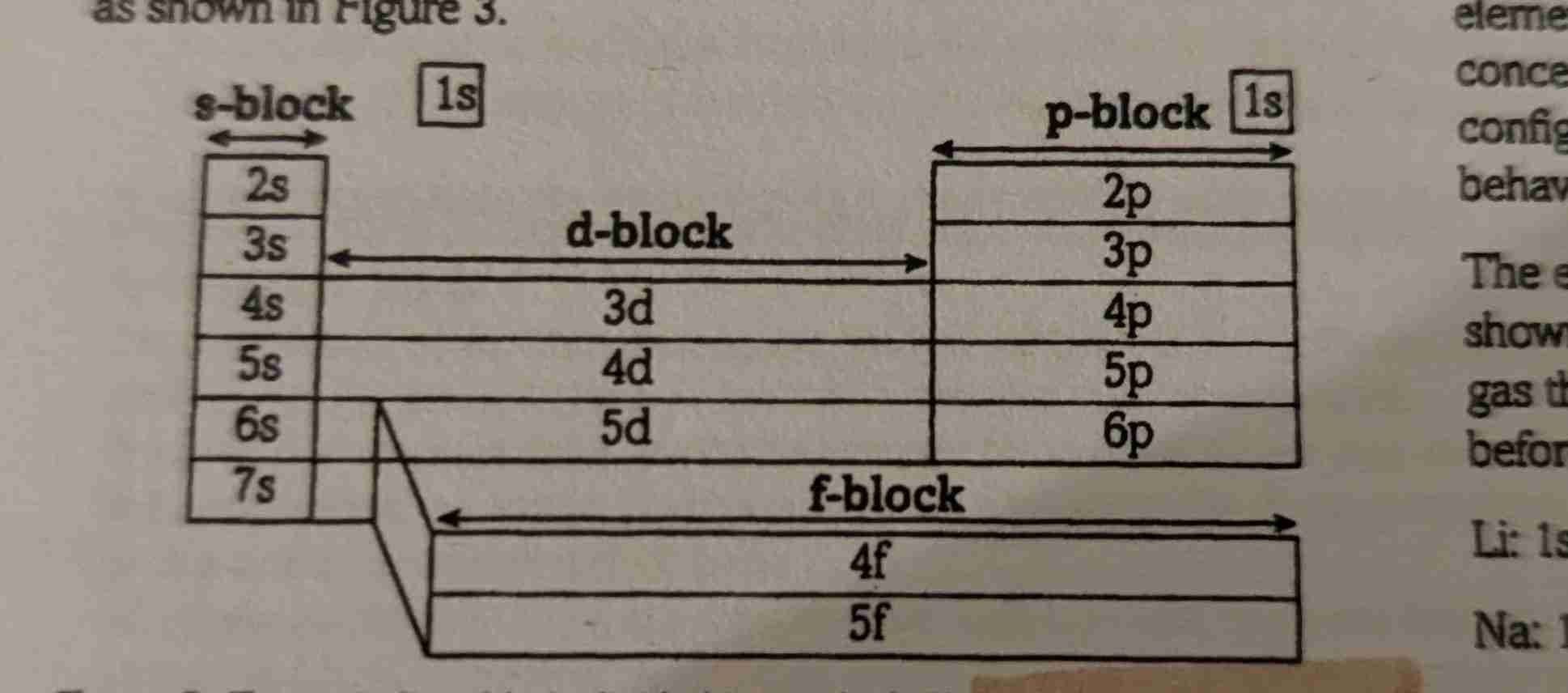
What are the sub shell groups of the periodic table?
Definition of ionisation energy
The amount of energy needed for the removal of 1 mol of electrons from 1 mol of gaseous atoms
What's the first ionisation energy?
The amount energy needed for the removal of 1mol of electrons from 1mol of gaseous atoms.
Ioniseation energy is always calculated in the gaseous state
How does atomic radius affect the nuclear attraction experienced by an electron?
The larger the atomic radius, the smaller the nuclear attraction experienced by the outer electrons.
How does nuclear charge affect the nuclear attraction experienced by an electron?
The higher the nuclear charge, the larger the attractive force on the outer electrons
How does electron shielding or screening affect the nuclear attraction experienced by outer electrons ?
Inner shells of electrons repell the outer shell electrons as they are negative which is called electron shielding / screening
The more inner shells there are, the larger the shielding effect and the smaller the nuclear attraction experienced by the outer electrons
Why are each successive ionisation energies higher than the one before?
As each electron is removed there's less repulsion between remaining electrons, so the shell will be drawn closer to the nucleus decreasing the distance of each electron to the nucleus therefore increasing nuclear attraction and more energy is needed to remove each successive electron.
Definition of relative atomic mass?
Weighted mean mass of an atom of an element compared with 1/12 th mass of an atom carbon - 12
Relative isotopic mass definition
The mass of an atom of an isotope compared with 12th mass of a carbon-12 atom
What's the trend in first ionisation energy going down a group?
First ionisation energy decreases going down a group
1) there are more protons so a greater nuclear charge increasing nuclear attraction
2) atomic radius increases as there are more electron shells therefore
3) shielding increases as you go down as there are more electron shells therefore a greater repulsion force caused by inner electrons decreasing nuclear attraction
Overall nuclear attraction decreases and first ionisation energy decreases
Explain the exceptions to the increase in first ionisation energy going across p2 and p3
In p3 the first ionisation energy decreases from Mg to AL and in p2 it decreases from Be to B as there's a change in subshell, AL has a 3p subshell and B has a 2p subshell so less energy is needed to remove an electron from the 2P or 3p orbital than the 2s or 3s subshell of Be and AL.
The first ionisation energy decreases from P to S in P2 and from N to O in p3 as S and O have a pair of electrons in one of their 3p/2p orbitals, the repulsion between them makes an electron easier to remove.
Explain the trend of first ionisation energy going across a period
1) nuclear charge increases as there are greater no of protons, this increases nuclear attraction
2) and decreases atomic radius which also increases nuclear attraction
3) shielding stays the same, same number of electrons shells so the same repulsion force caused by inner electrons.
Obe nuclear attraction decreases and ionisation energy decreases