Quantum Numbers
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
20 Terms
Quantum Number
A solution to the Schrodinger equation that specifies the properties of an atomic orbital and the electrons found in that orbital.
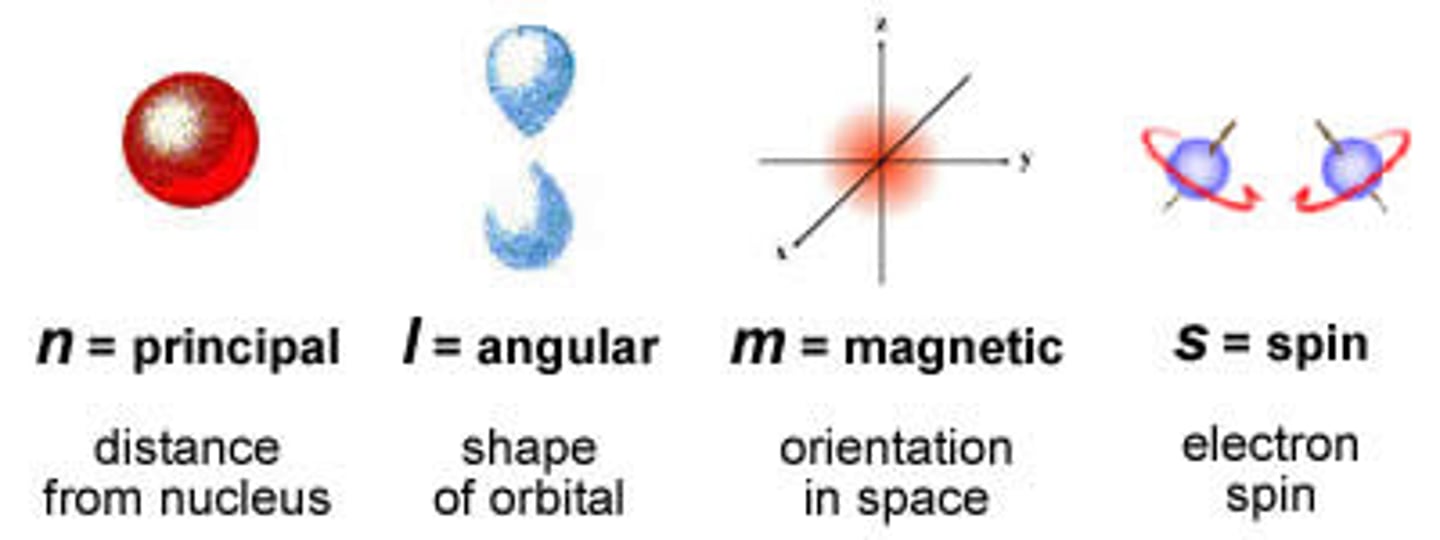
Principle Quantum # (symbol)
n
Angular Momentum Quantum # (symbol)
l
Magnetic Quantum # (symbol)
m
Spin Quantum # (symbol)
s
Principle Quantum # (definition)
Indicates the main energy level occupied by the electron.
Angular Momentum Quantum # (definition)
Indicates the shape of the orbital.
Magnetic Quantum # (definition)
Indicates the orientation of an orbital around the nucleus.
Spin Quantum # (definition)
Indicates the direction an electron rotates in its orbital.
What are the allowable values for n?
Any positive integer, from 1 to n.
What are the allowable values for l?
Any positive integer in the range [0, ..., n-1].
What are the allowable values for m?
Any integer in the range [-l, ..., +l].
What are the allowable values for s?
-1/2 and +1/2 only.
Orbital
A three-dimensional region around a nucleus that indicates the probable location of an electron, defined by specific values of n, l, and m.
Sublevel/Subshell
All similarly shaped orbitals that share the same values for n and l.
Energy Level/Shell
All orbitals that share the same value for n only.
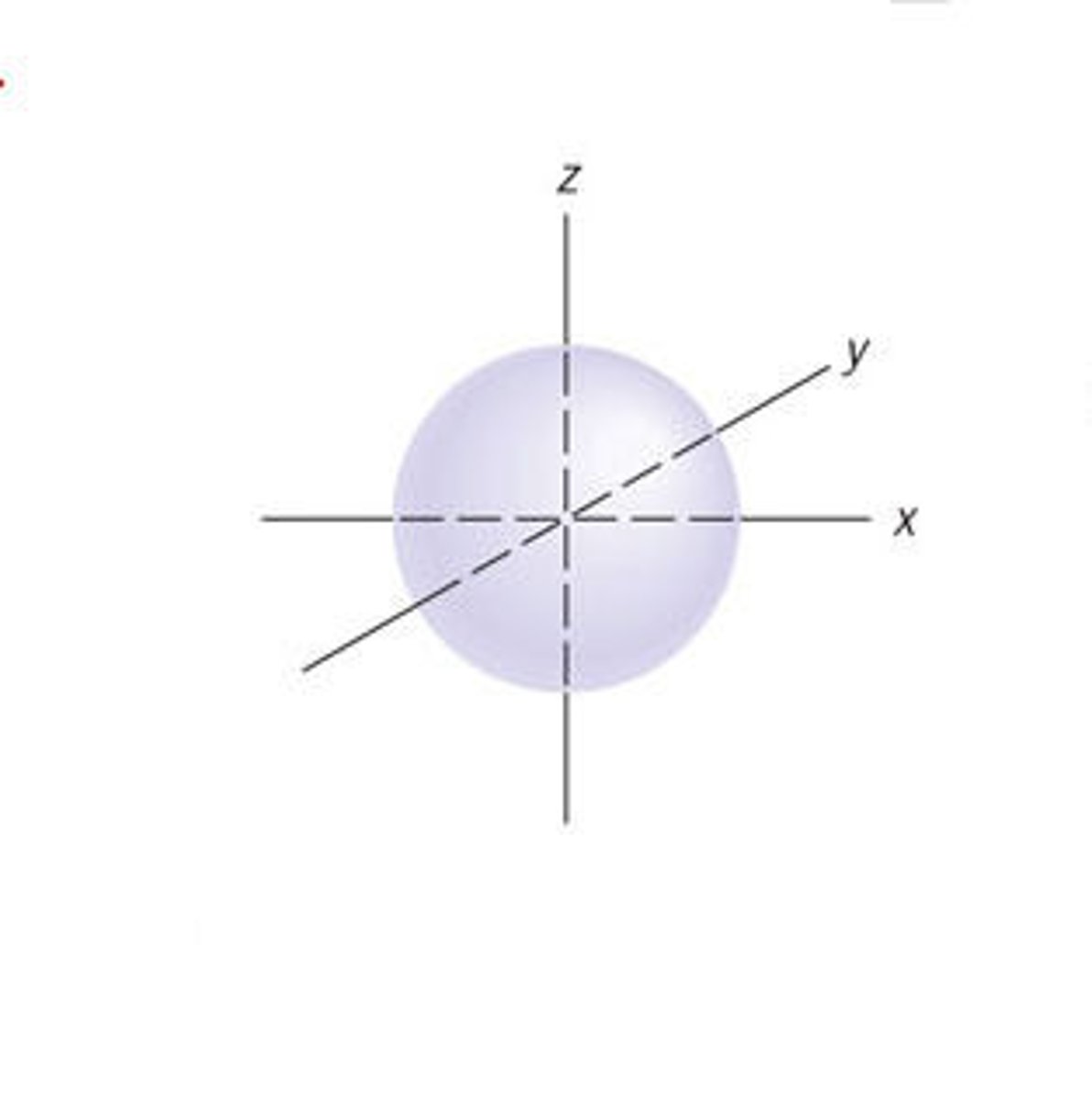
s-orbital
A spherical orbital defined by l=0
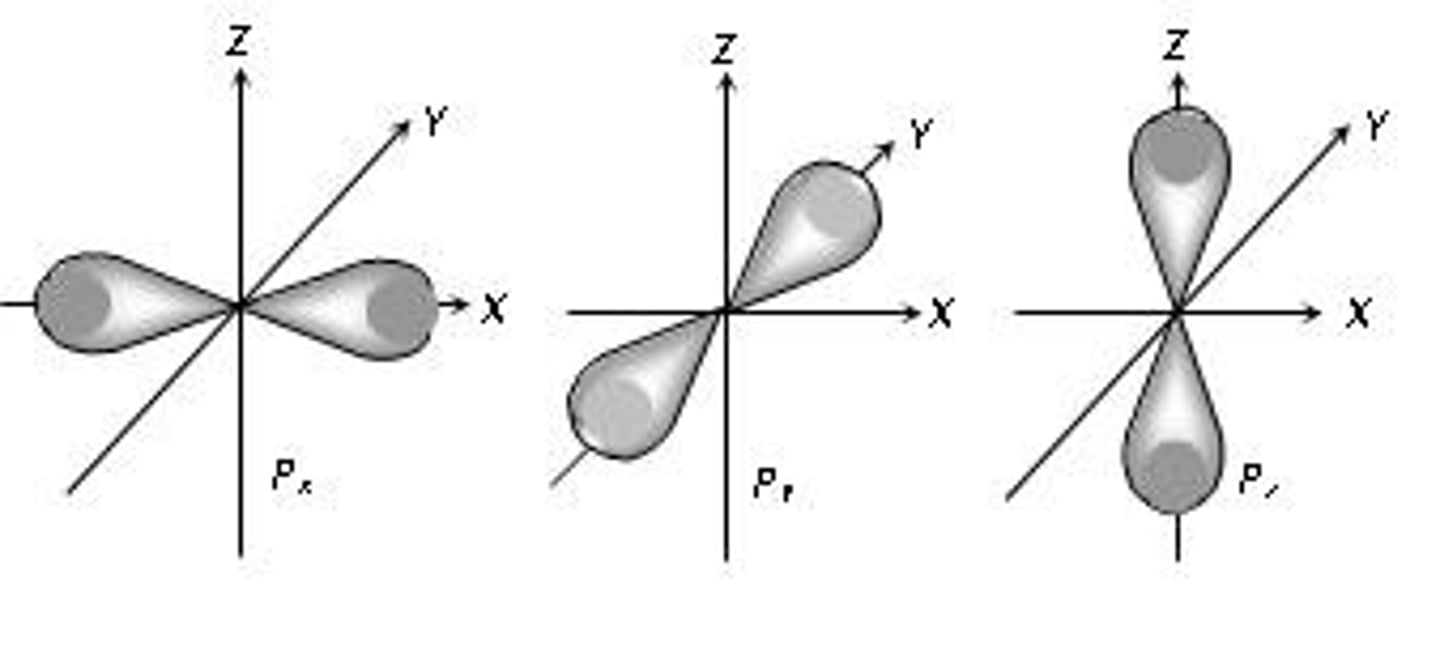
p-orbital
A dumbbell-shaped orbital defined by l=1
d-orbital
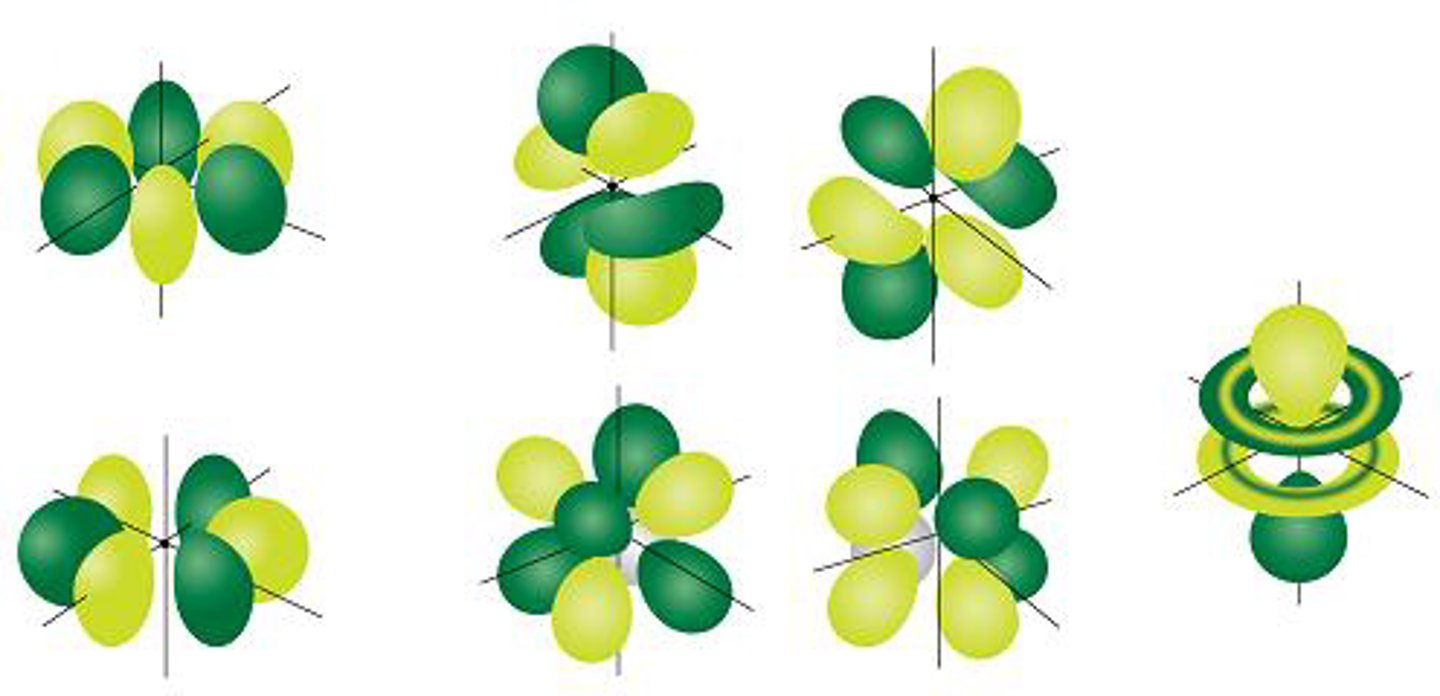
A cloverleaf-shaped orbital defined by l=2
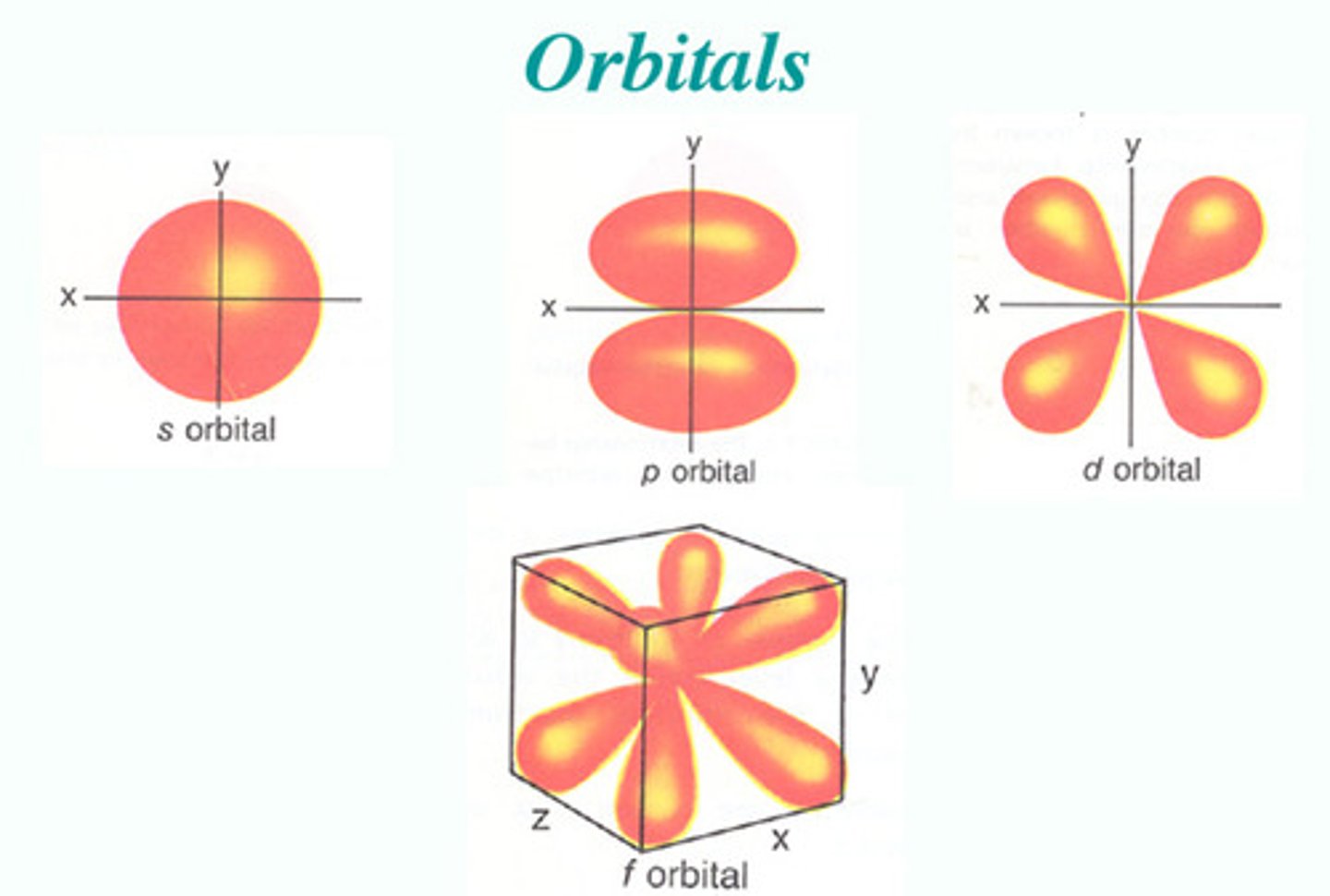
f-orbital
A complex orbital defined by l=3