R1.3.1/R1.3.2 Combustion
1/7
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
8 Terms
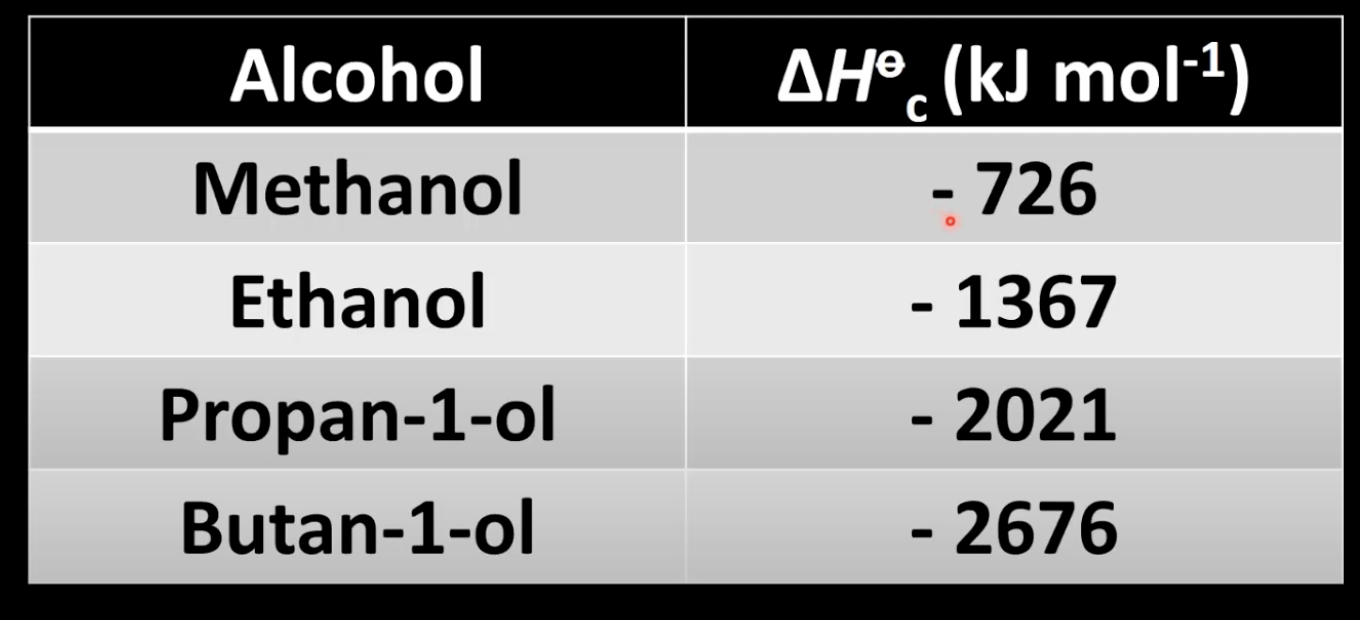
Alkanes react with oxygen to form CO₂ and H₂O.
Exothermic reaction with negative enthalpy change.
Releases large amounts of heat.
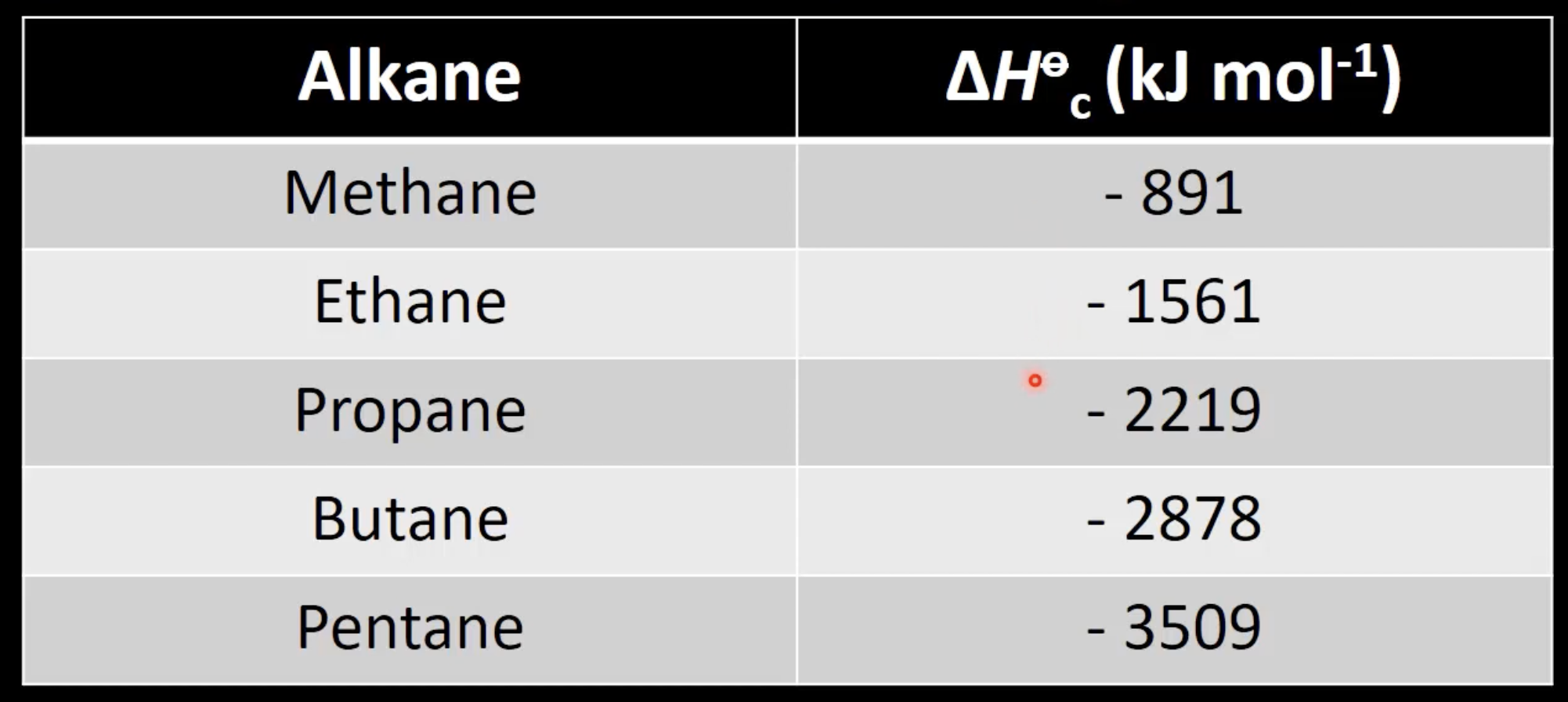
Enthalpy trend in alkane combustion
Enthalpy values are negative and increase in magnitude with carbon chain length.
More CO₂ and H₂O produced with longer chains.
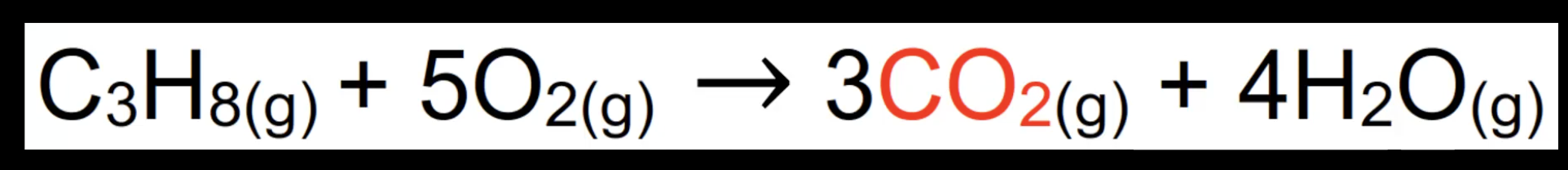
Complete combustion of alkanes
Occurs with excess oxygen.
Produces carbon dioxide and water.
Example: C₃H₈ + 5O₂ → 3CO₂ + 4H₂O.
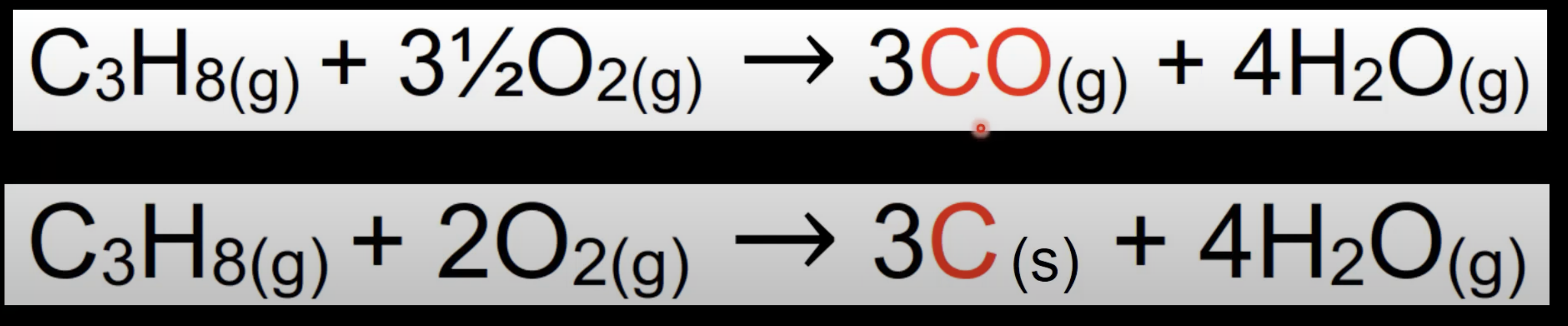
Incomplete combustion of alkanes
Occurs with limited oxygen.
Produces CO and/or C with water.
Less efficient, more pollutants formed.
Occurs in excess oxygen.
Produces CO₂ and H₂O.
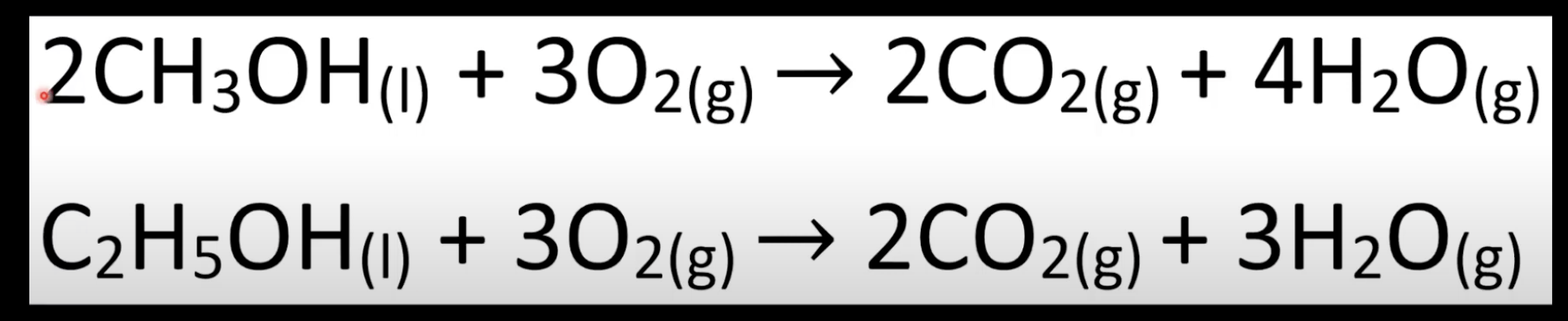
Occurs with limited oxygen.
Produces CO or C with water.
Less energy released than complete combustion.
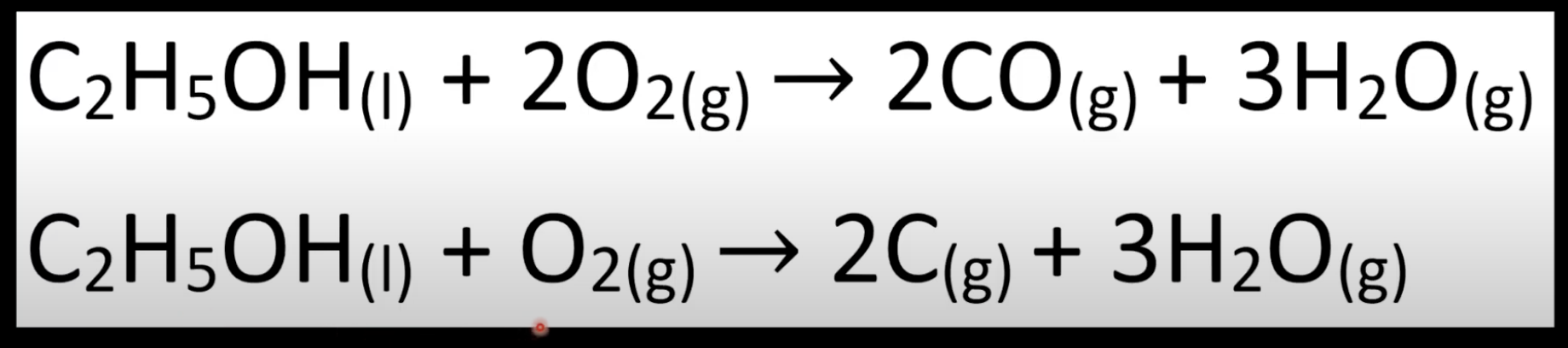
Values are negative and more exothermic with more carbon atoms.