Atomic structure and electronic configuration
1/9
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
10 Terms
What is atomic structure?
The structure of the atom and how it is organised with the shells and its nucleus.
What is electronic configuration?
The way that electrons are arranged in an atom. E.g For Calcium the electronic configuration is 2,8,8,2 as the atomic number is 20 and that is what the number of electrons in the configuration add up to.
What is an isotope?
Atoms of the same element with different number of neutrons.
They have the same chemical properties and different physical properties
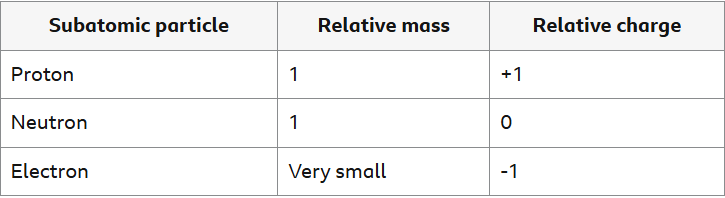
What are the relative charges and mass’ of protons, electrons and neutrons?
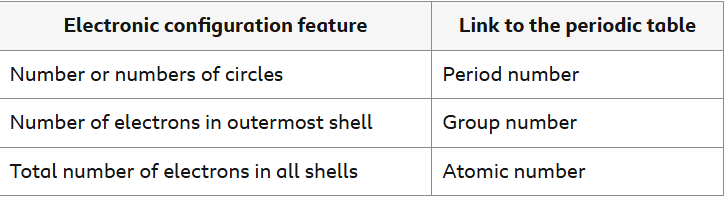
What are the links to an elements electronic configuration and its position on the periodic table?
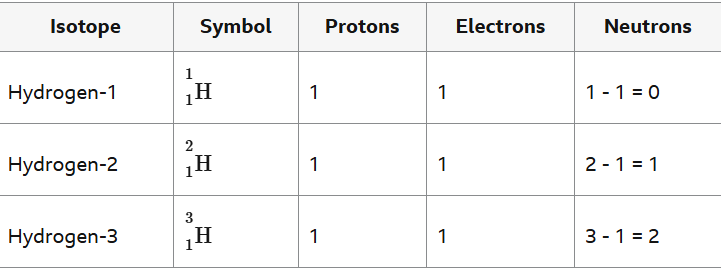
Describe the link between sodium and its positioning on the periodic table.
it is in period 3 (number of circles / shells)
it is in group 1 ( number of electrons on the outer shell)
has an atomic number of 11 ( total number of electrons in all shells, 2+8+1=11)
What do you do to find the neutron charge?
Subtract the atomic number from the atomic mass. Neutron charge = atomic mass - atomic number.
What is the maximum structure of electronic configuration?
2,8,8
What is an isotope named after?
An isotope is named after the element and the mass number. e.g carbon -12 is an isotope of carbon with a mass number of 12.
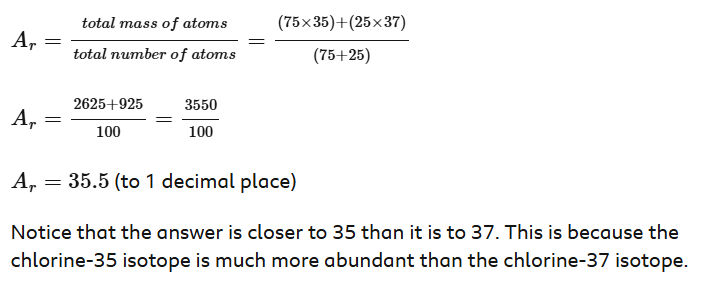
Calculating relative atomic mass - chlorine
The abundance of chlorine-35 is 75% and the abundance of chlorine-37 is 25%.
Meaning, in every 100 chlorine atoms, 75 atoms have a mass number of 35, and 25 atoms have a mass number of 37.