subatomic particles
1/11
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
12 Terms
relative electrical charges of subatomic particles
proton +1
electron -1
neutron 0
why don’t atoms have an overall charge
In an atom, the number of electrons is equal to the number of protons in the nucleus
what do atoms of the same element have in common
the same amount of protons
what is radius of atom
having a radius of about 0.1 nm (1 x 10-10 m).
what is the radius of nucleus
The radius of a nucleus is less than 1/10 000 of that of the atom (about 1 x 10-14 m).
where is most of mass in the atom
Almost all of the mass of an atom is in the nucleus.
relative mass of subatomic particles
proton 1
neutron 1
electron very small
what makes the mass number
The sum of the protons and neutrons in an atom is its mass number.
isotope
Atoms of the same element with different numbers of neutrons but same number of protons and electrons
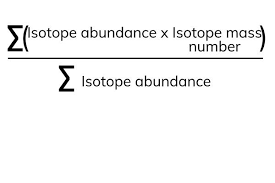
what is the relative atomic mass of an element
is an average value that takes account of the abundance of the isotopes of the element
formula for relative atomic mass
which shells do electrons occupy first
the lowest available energy levels