Naming Acids and Bases
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
40 Terms
LiOH
Lithium Hydroxide
NaOH
Sodium Hydroxide
KOH
Potassium Hydroxide
Zn(OH)₂
Zinc Hydroxide
Hg(OH)
Mercury(I) Hydroxide
Mn(OH)₃
Manganese (III) Hydroxide
Mn(OH)₂
Manganese(II) Hydroxide
Mg(OH)₂
Magnesium Hydroxide
Pb(OH)₄
Lead(IV) Hydroxide
Pb(OH)₂
Lead(II) Hydroxide
Fe(OH)₃
Iron(III) Hydroxide
Ir(OH)₃
Iridium(III) Hydroxide
NH₄OH
Ammonium Hydroxide
Ba(OH)₂
Barium Hydroxide
Ca(OH)₂
Calcium Hydroxide
Fe(OH)₂
Iron(II) Hydroxide
Mg(OH)₂
Magnesium(II) Hydroxide
Al(OH)₃
Aluminum Hydroxide
HCl
hydrochloric acid
HNO₃
nitric acid
HClO₃
chloric acid
HBr
hydrobromic acid
HClO₂
chlorous acid
HNO₂
nitrous acid
H₂S
hydrosulfuric acid
H₂SO₄
sulfuric acid
H₂SO₃
sulfurous acid
HF
hydrofluoric acid
H₃PO₄
phosphoric acid
HI
hydroioidic acid
H₃PO₃
phosphorous acid
HC₂H₃O₂
acetic acid
H₂Se
hydroselenic acid
H₂CO₃
carbonic acid
H₂SO₄
Sulfuric Acid
Acid
conducts electricity in solution, tastes sour, feels watery, reacts vigorously w/metals to produce H₂ gas, clear in phenolphthalein, turns blue litmus paper red

Base
conducts electricity in solution, tastes bitter, feels slippery, does not react w/metals, pink in phenolphthalein, turns red litmus paper blue

Acidic Solution
exists when pH < 7
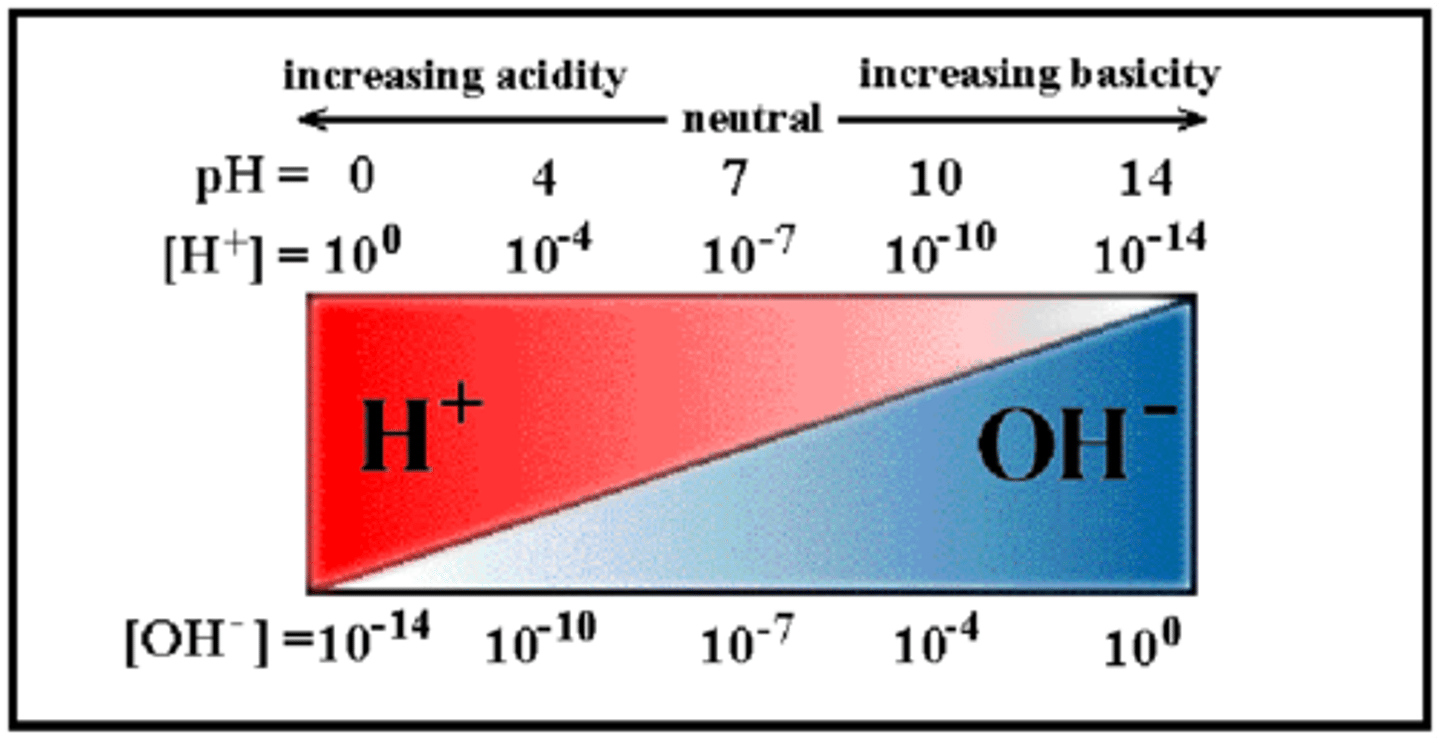
Basic Solution
exists when pH > 7
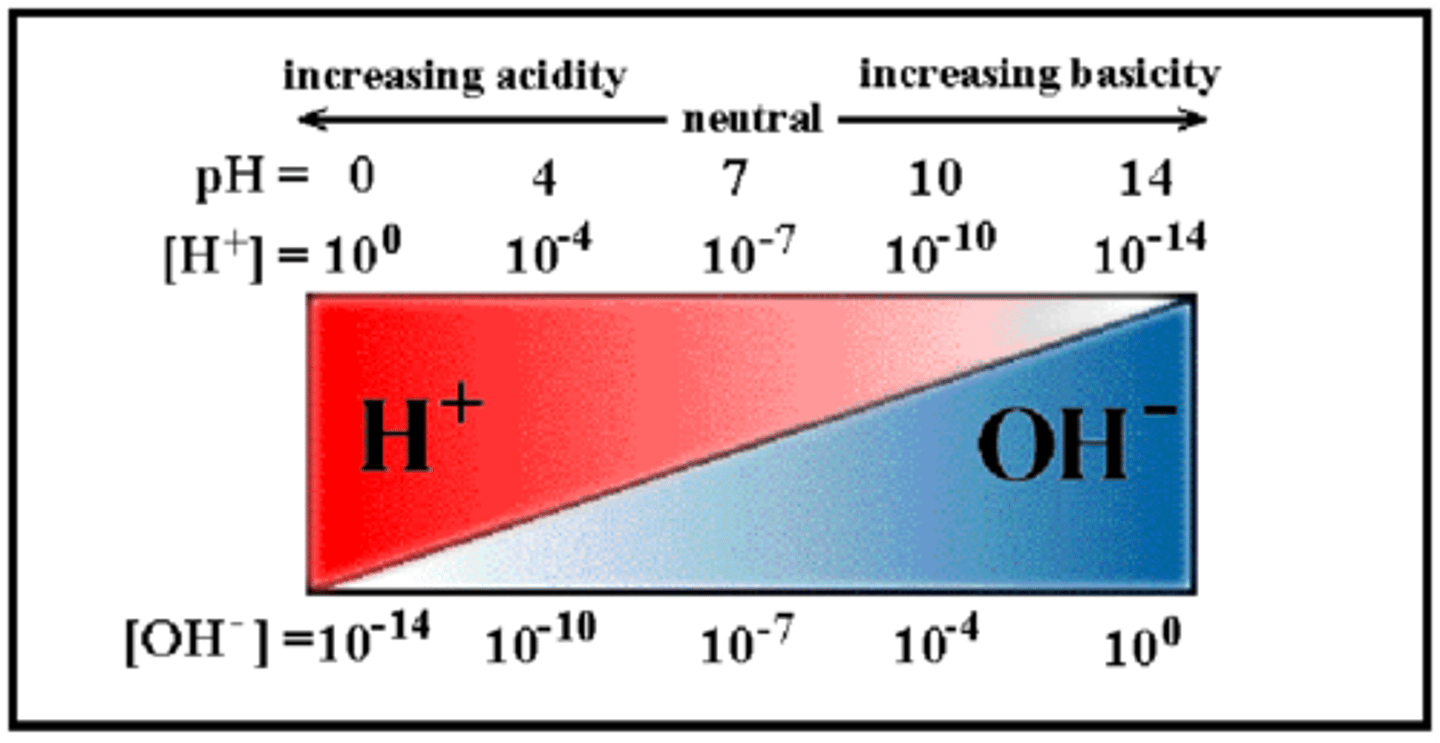
hydrophosphoric acid
H₃P