BBMB 2210 Exam 2
1/24
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
25 Terms
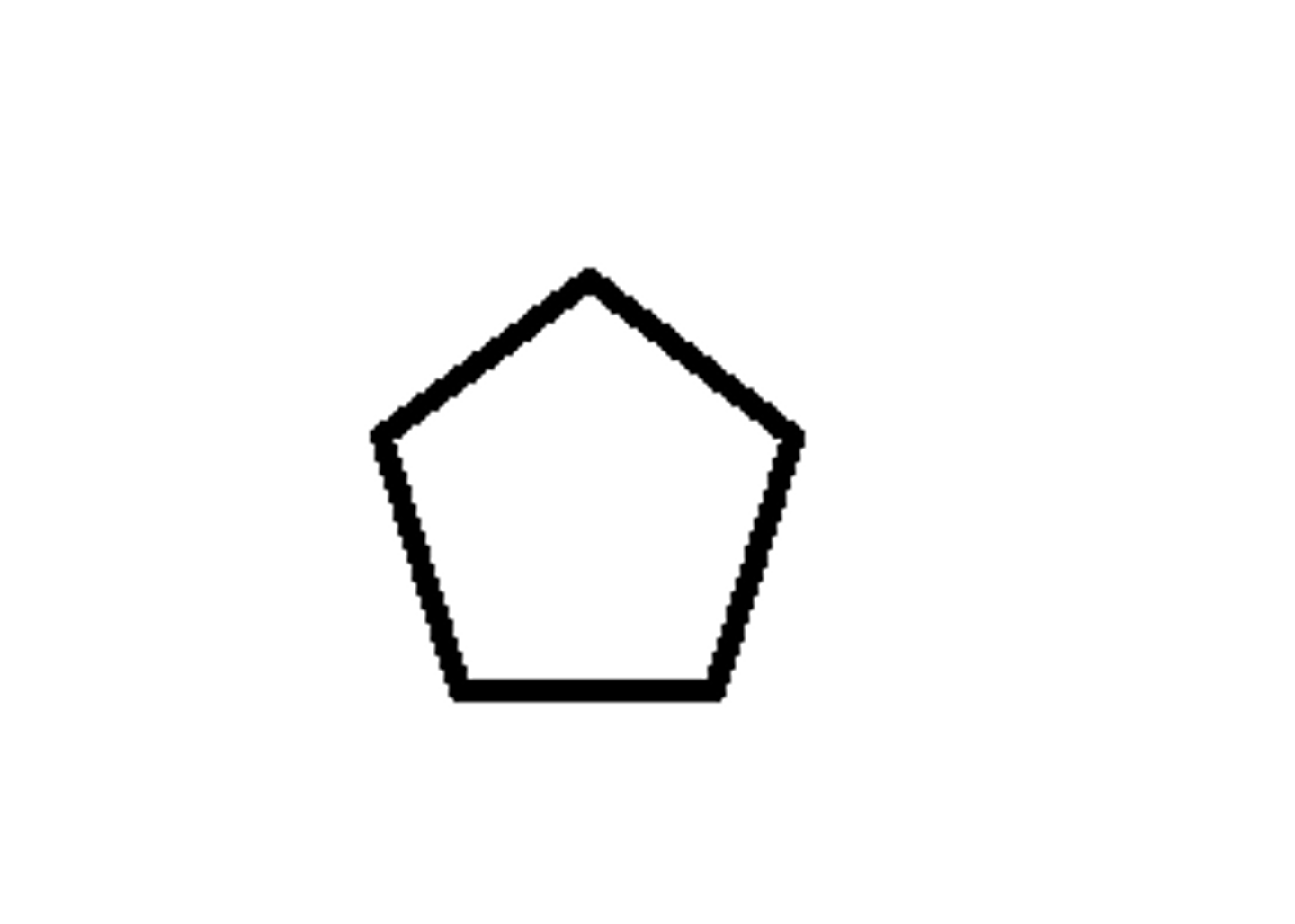
What is the name of the following compound?
cyclopentane
In the combustion reaction of a compound like octane, which of the following
compounds are products?
I) Carbon dioxide
II) Water
Both I and II
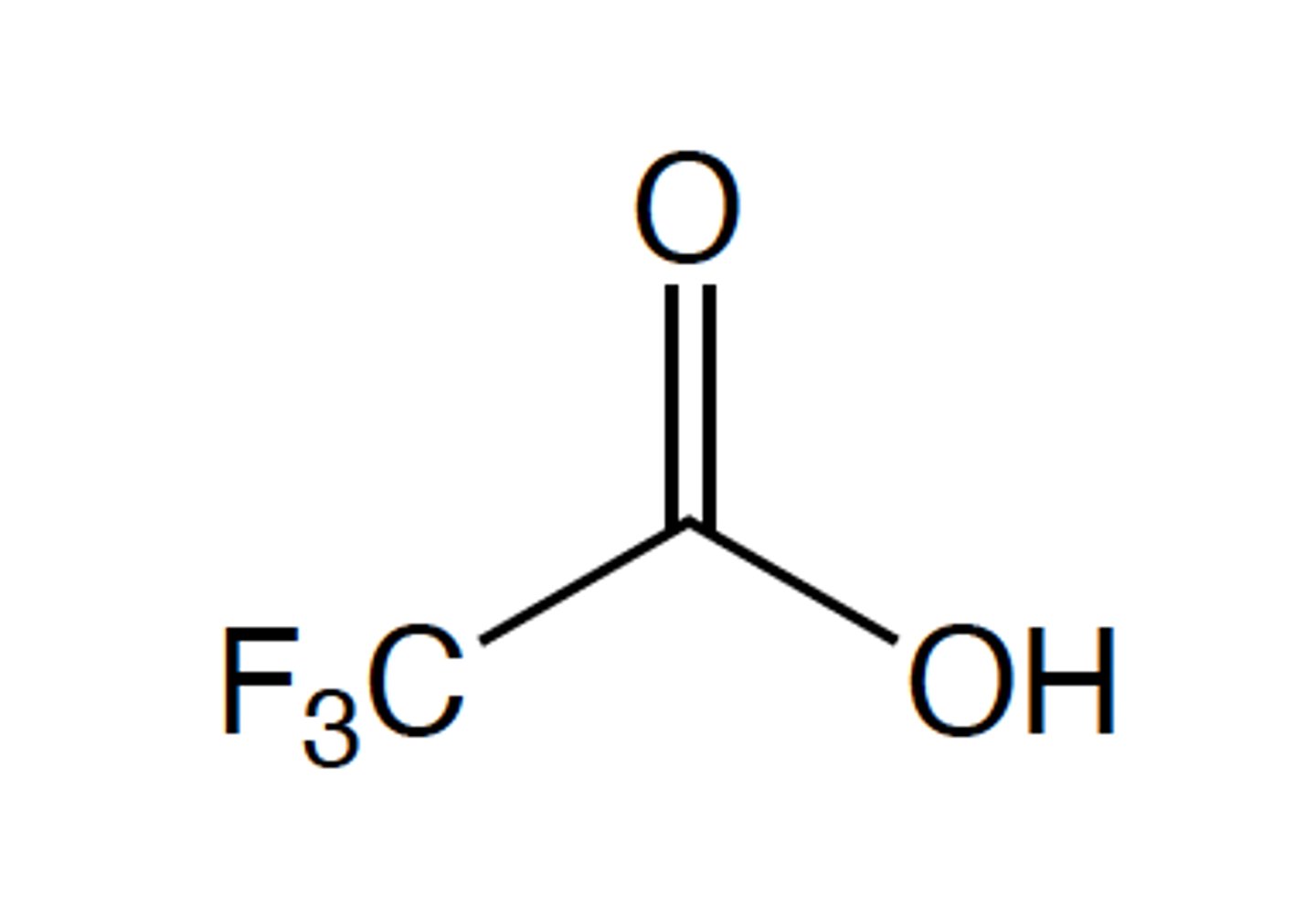
The reason that best explains why this compound is more acidic than acetic acid is which of the
following? Note - although we have not talked about compounds containing fluorine atoms in
class, recall that fluorine is even more electronegative than oxygen.
Inductive effects
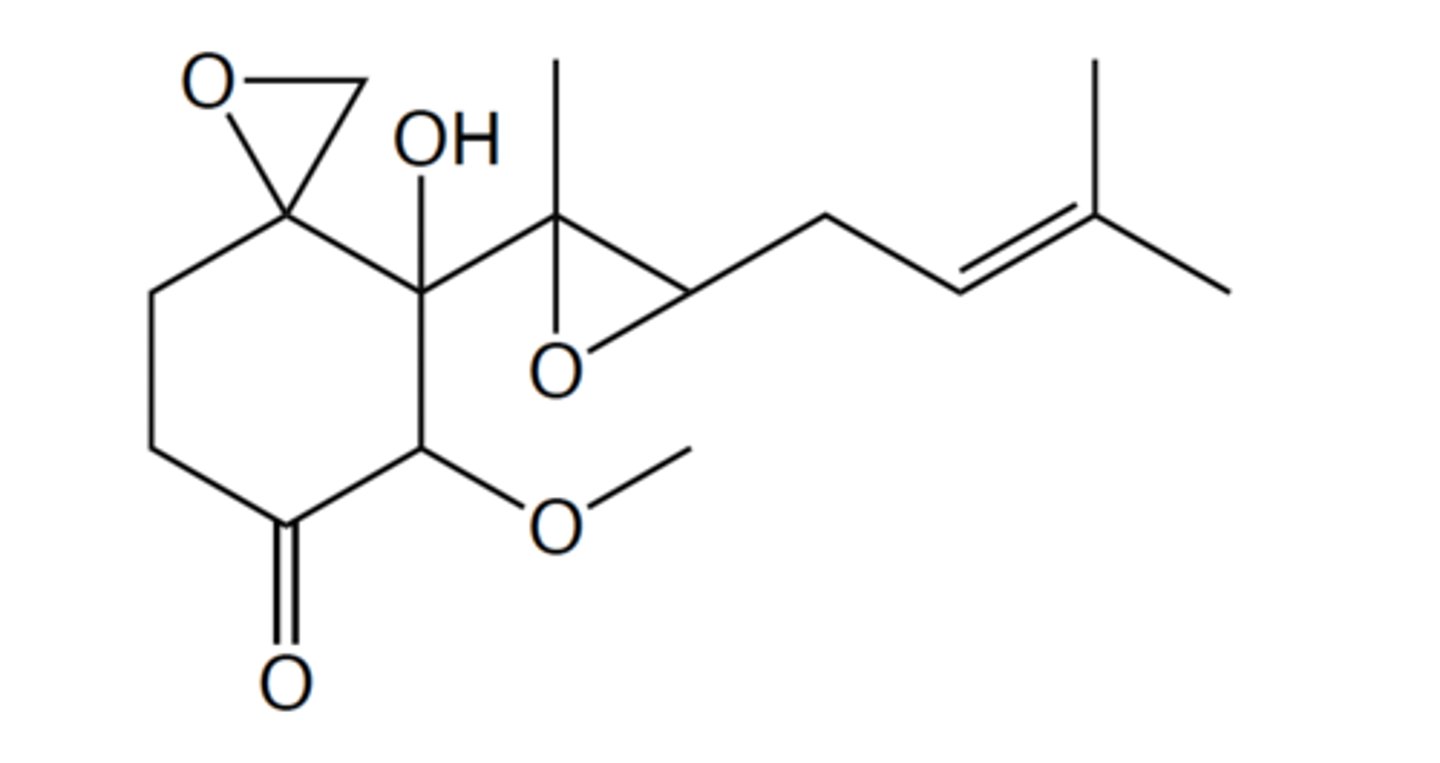
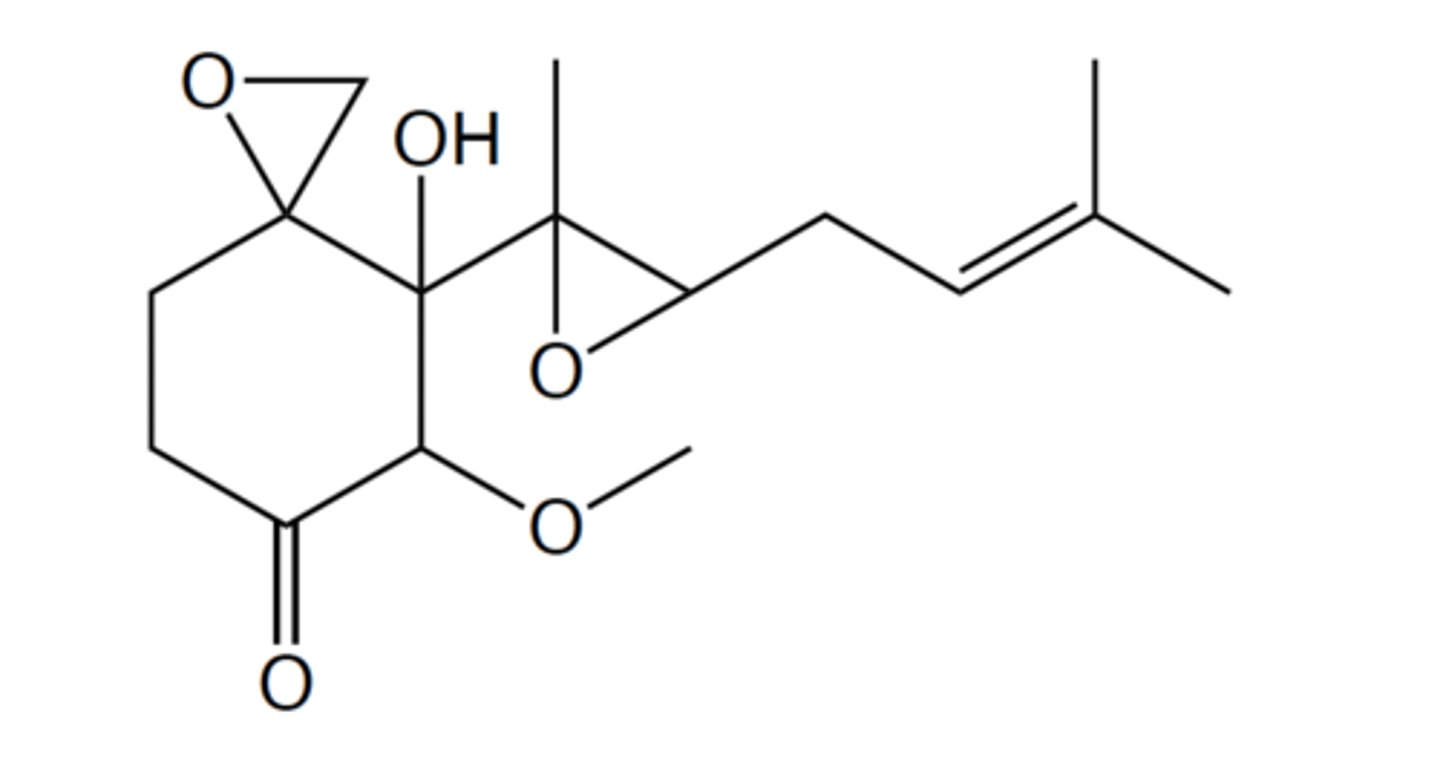
The following is a naturally occurring compound that can be isolated from a certain
species of fungus:
How many hydrogens does this compound have? (Drawing the structure on a piece of paper
could be helpful for counting the hydrogens.)
24
For the compound shown in the previous question, which of the following
functional groups is not present:
Ester is not present
Continued from the previous question. Is the molecule conjugated?
Molecule is not conjugated
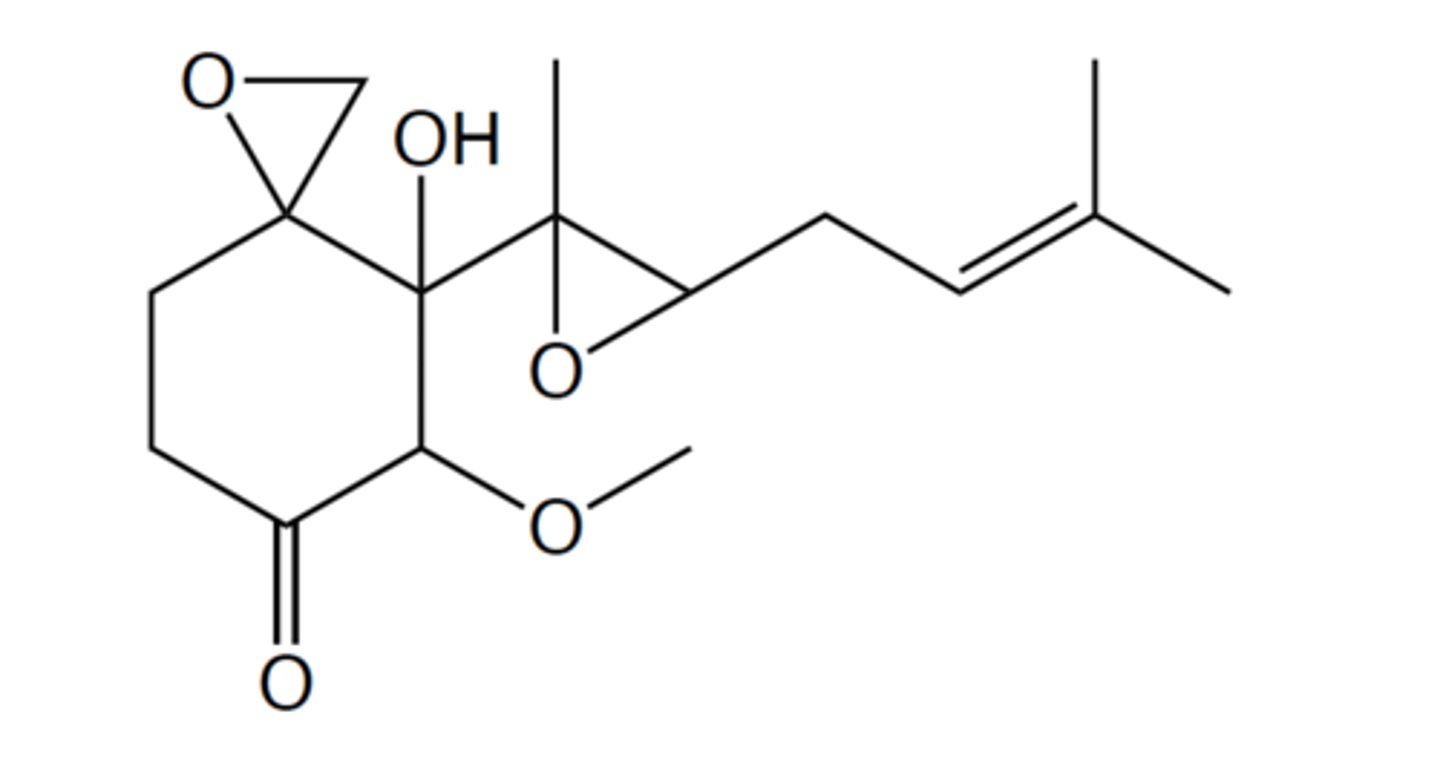
Continued from the previous question. The molecule has a total of five chiral
centers. How many total stereoisomers of the compound are there?
32
Suppose one of the functional groups in the protein made a hydrogen bond to the oxygen of
the portion of the molecule shown above. Which of the following statements is true? The
oxygen in the three-membered ring can serve as a…
I) hydrogen bond acceptor
II) hydrogen bond donor
Only I is true
Continued from the previous question. Let’s consider the protein functional group
that forms a hydrogen bond to the oxygen in the compound’s three-membered ring shown in
the previous question. In the protein, which of the following functional groups could form that
hydrogen bond?
Amide group
The three-membered ring with an oxygen shown two questions ago is very
reactive. Which of the following is a likely reason for the instability of the ring structure?
Bond strain
In the mechanism shown on the left-hand side, the curved arrow between the nitrogen and the
carbon indicates where the _____ from the _____ end up.
electrons, nitrogen
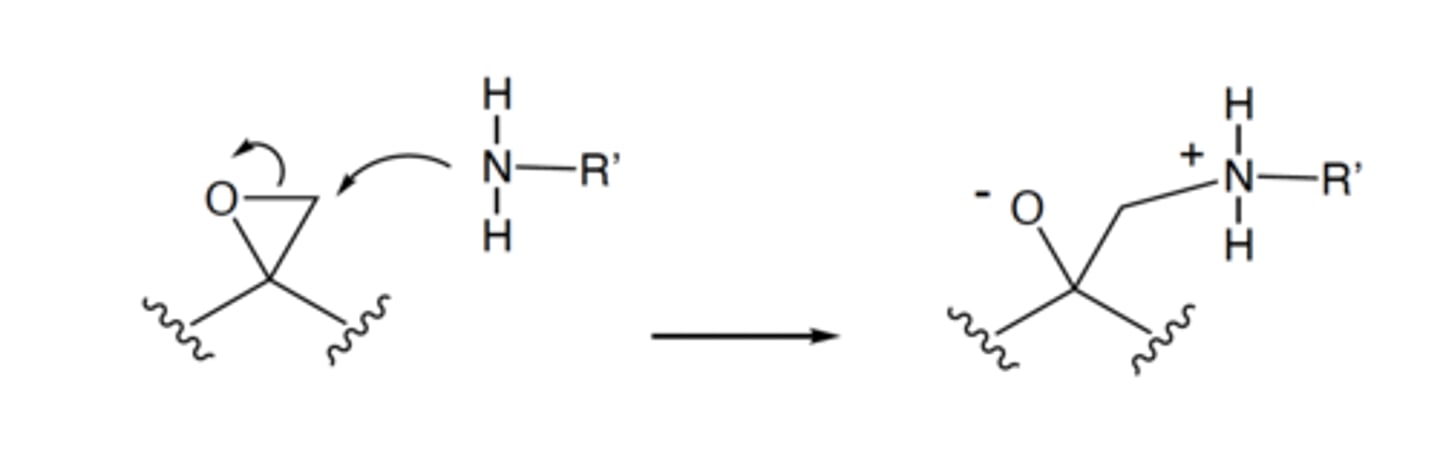
Continued from the previous question. In the mechanism shown in the previous
question, the straight arrow between the structures on the left and the structures on the right
indicate that:
The reaction is effectively irreversible
The reaction occurs rapidly
The structures on the left and right are in equilibrium
The structures on the left and right are resonance structures
The reaction is effectively irreversible
Continued from the previous question. The reaction from two questions earlier
would be slow because the covalently-linked product shown is high in energy. Analogous to
how alcohol addition to aldehydes and ketones can be sped up, formation of the covalentlylinked
product can be sped up by using an alternative mechanism that involves:
Carboxylic acid interconversion reactions
Combustion reactions
Electron transfer reactions
Proton transfer reactions
Proton transfer reactions
In a population of ethane molecules, ethane can adopt a staggered or an eclipsed conformation (amongst others). The relative number of ethane molecules in the eclipsed versus staggered conformation is dictated by which of the following:
I) The difference in energy between the staggered and eclipsed conformation
II) The temperature the ethane molecules are at
Both I and II
The functional group that includes the sulfur atom is an example of a:
An alcohol
An ether
A thioether
A thiol
A thiol
The compound has an orange appearance.
The reason the molecule is colored is that the _____ conjugation in the molecule allows it to
absorb light in the visible spectrum. The reason it appears orange, specifically, is that it _____
orange light.
absence of, absorbs
absence of, does not absorb
extensive, absorbs
extensive, does not absorb
extensive, does not absorb
In this isomerization reaction, there is a rotation around the double bond as shown. Light
provides energy for this process because in going from the starting material to the product,
there is an intermediate (not show) that is _____ in energy than the starting material. The
energy of rotating around the carbon-carbon double bond is _____ that for rotation around a
carbon-carbon bond in a compound like butane.
lower, lesser than
lower, greater than
higher, lesser than
higher, greater than
higher, greater than
If this compound is deprotonated, it would have a _____ charge, and in this deprotonated form
we would expect it dissolves in a non-polar solution _____ than in its protonated form.
positive, less
positive, more
negative, less
negative, more
negative, less
Continued from the previous question. Inside a cell buffered at pH 7, we would
expect the compound shown in previous question to be predominantly in the form of the
_____.
Acid anhydride
Carboxylate
Carboxylic acid
Ketone
Carboxylate
The compound on the left accepts two electrons to become the compound on the right. This is
an example of _____ reaction, and in this reaction the compound on the left is _____ to
become the compound on the right.
an electron transfer, oxidized
an electron transfer, reduced
a proton transfer, oxidized
a proton transfer, reduced
electron transfer, reduced
Of the two resonance structures, we would expect the one on the _____ to contribute more,
because _____ is more electronegative.
right, oxygen
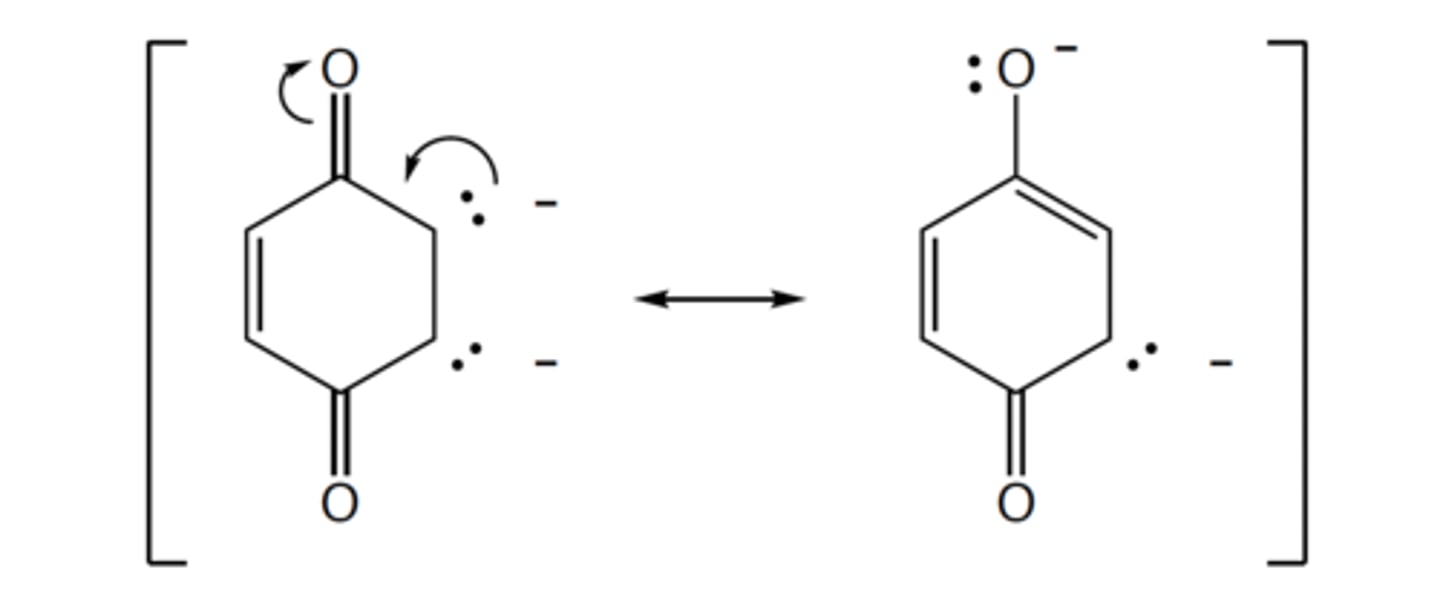
Formally this makes the molecule on the left _____. In relation to the resonance structures
shown in the previous question, the molecule on the right is _____ resonance structure.
an acid, an additional
an acid, not an additional
a base, an additional
a base, not an additional
a base, not an additional
As an example of a hydrolysis reaction, when an acid anhydride compound
encounters ______ the acid anhydride _____
a water molecule, joins with another acid anhydride
a water molecule, splits into two compounds
an alcohol molecule, joins with another acid anhydride
an alcohol molecule, splits into two compounds
a water molecule, splits into two compounds
Based on their relative stabilities, which of these statements about interconvertion
of an ester to another carboxylic acid derivative is true?
I) Adding an amine to the ester will lead to significant production of an amide.
II) Adding a carboxylate to the ester will lead to significant production of an acid anhydride.
I only
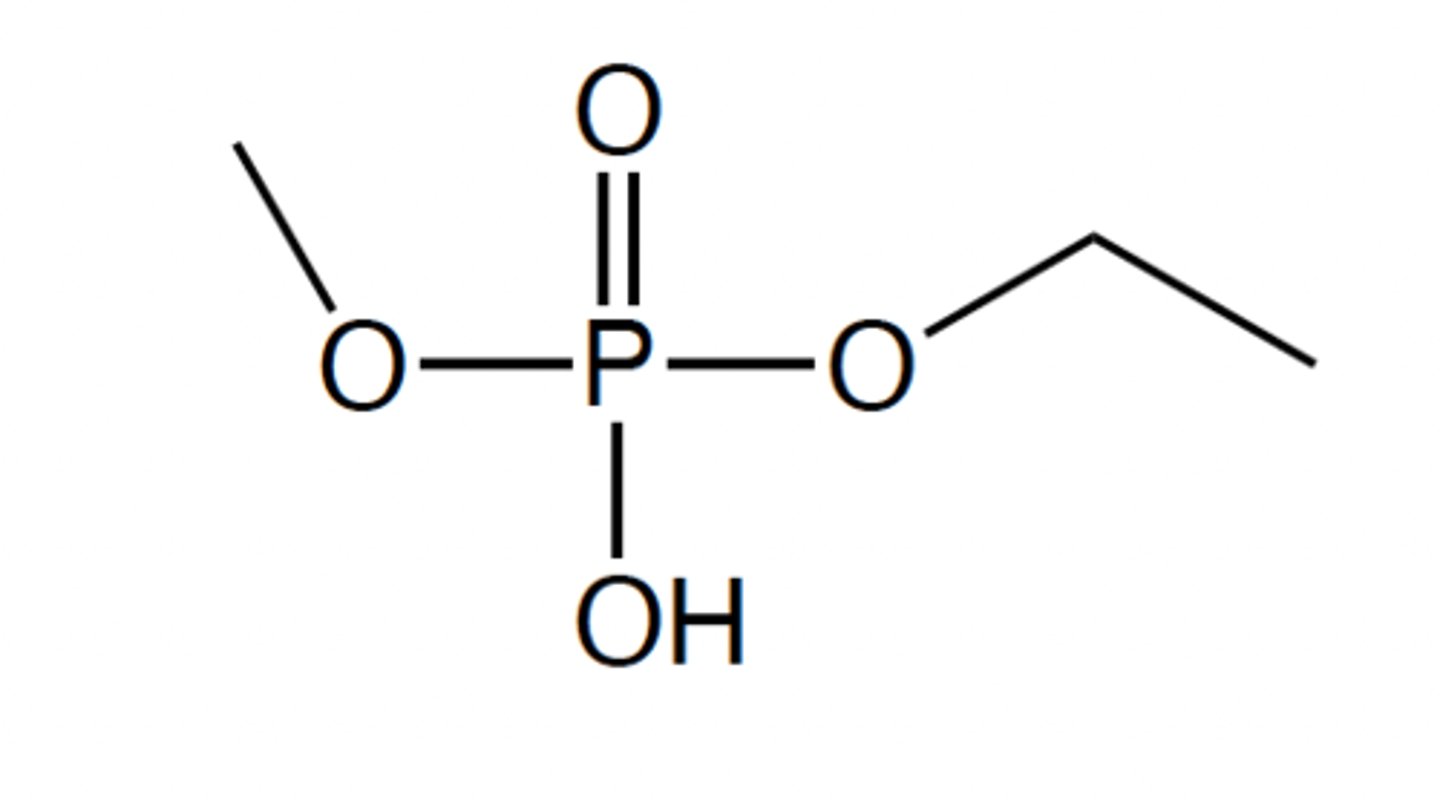
The following compound is an example of what?
A phosphate ester