Molecular Polarity
1/3
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
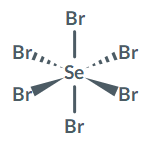
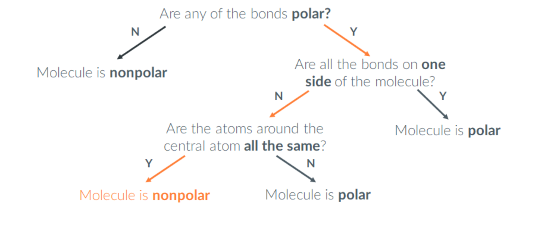
Is SeBr6 polar or nonpolar?
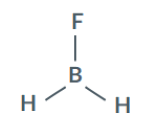
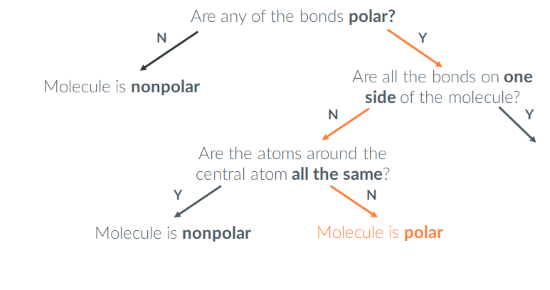
Is BH2F polar or nonpolar?
A component of crude oil is propane, pictured below. Using the knowledge that polar molecules don’t mix with nonpolar molecules, explain why crude oil sits on top of water.
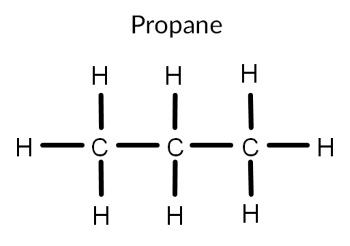
The difference in electronegativity between carbon and hydrogen is less than 0.5. This means that we consider the C-H bond to be nonpolar. As propane only consists of C-H bonds, we say that propane itself is nonpolar.
On the other hand, the difference in electronegativity between oxygen and hydrogen is more than 0.5. This means that O-H bonds are polar. They are also on one side of the molecule, so their dipoles don’t cancel out. This means that water is polar.
As propane is nonpolar, and water is polar, they do not mix. This explains why crude oil sits on top of water.
ffff