Energy Methods - Block 1
1/86
Earn XP
Description and Tags
1.1 - Intro to thermodynamics (1-31) 1.2 - Energy transfer by heat (32-48) 1.3 Heat Capacity and energy Storage (49 - 54) 1.4 First Law of thermodynamics (55-84)
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
87 Terms
What is thermodynamics?
The theory of the relationship between heat and energy. It is the study of the effects of work, heat and energy on a system.
What is a system?
A system is self contained in an area and is the process or value that we are interested in.
What is the surroundings?
the regions outside the system.
What is a boundary?
the surface dividing the system from the surroundings. The boundary can be fixed or movable
What three type of systems can there be?
Open – mass and energy can transfer between the system and the surroundings
Closed – energy can transfer between the system and surroundings, but NOT mass
Isolated – neither mass or energy can transfer between the system and surrounding
What is Steady Flow Process (Open Systems)?
Working fluid flows steadily and uniformly eg. Boilers, turbines, compressors, heat exchangers
What is a Non-Flow Process (Closed Systems)?
The volume of the system may change, no fluid crosses the boundary, energy may be transferred across the boundary eg. Internal combustion engines
What is thermal equilibrium?
the temperature is the same throughout the system
What is mechanical equilibrium?
there is no change in pressure with time
What is a state function?
a function defined for a system relating several state variables, eg temperature, pressure and volume
Why are work and heat not properties?
Work and heat transfer are not properties because they are dependent on the path that is taken to change from one state to another. They are known as path functions. A system does not possess heat or work. These functions are either input or output during a change in the system.
What is a process?
A process describes the path between states
What is a path?
a series of equilibrium states which a system passes through during a process.
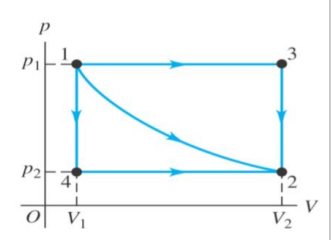
What are the three different paths that could be taken on this P-V diagram?
1-3-2 A constant pressure process followed by a constant volume process
1-4-2 A constant volume process followed by a constant pressure process
1-2 A single process in which both pressure and volume are changing
What are the five process types?
Adiabatic – no heat transfer between the system and surroundings
Isobaric – constant pressure
Isothermal – constant temperature
Isochoric – constant volume
Polytrophic – pressure, volume and temperature all vary.
What is a cycle?
If a system undergoes a series of processes such that its final state is identical to its initial state, then it is said to have undergone a cycle.
Can a system actually be in equilibrium?
No system can be in complete equilibrium as it undergoes a real process. However, some processes are almost in perfect equilibrium throughout the process.
What doe it mean to for a process to be in ‘quasi-equilibrium’?
A quasi-equilibrium process is one in which the system only deviates from equilibrium by an infinitesimal amount.
(An example of a quasi-equilibrium process is a piston which compresses the gas very slowly. In this case, the force driving the compression would be infinitesimally small and the pressure changes would be infinitesimally small. - this type of process is known as a quasi-static process.)
What happens if two bodies are at different temperatures?
Heat is transferred from a body at a higher temperature to one at a lower temperature until both bodies are at the same temperature and thermal equilibrium is reached.
What is the zeroth law of thermodynamics?
When two bodies are in thermal equilibrium with a third body, they are also in thermal equilibrium with each other. therefore A=B=C
What is the difference between heat and temperature?
Heat is energy transferred due to a temperature difference. Heat is measured in Joules.
Temperature is a degree of hotness or coldness. Temperature is measured in degrees Celsius (°C) or Kelvin (K)
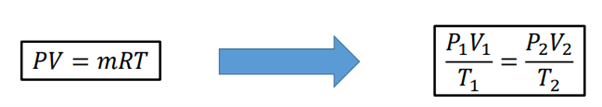
What is the ideal gas law?
PV = mRT (T must be in Kelvin)
What is the rate of heat transferred and/or the energy transfer by work measured in?
J/s = W (watts)W
What are the sign conventions when heat is lost or gained?
When heat is added to a system it is +ve
When heat is lost from a system it is –ve
What are the sign conventions when heat is lost or gained?
When work is done by a system it is +ve
When work is done to system it is –ve
(e.g During a gas expansion of a piston, the gas is doing work, work is output, this is +ve During a gas compression by a piston, work is being done to the gas, so this is -ve)
What is a heat engine?
A heat engine is a system which converts heat energy (𝑄 subscript 𝐻) to mechanical energy which can be used to do work (𝑊). During this process some of the heat is lost to the surroundings and some energy is unusable because of friction (𝑄 subscript 𝐿 ).
A heat engine can be represented as heat flow from a hot/high temperature reservoir (or source) to a cold/low temperature reservoir (or sink).
WHat are some examples of a heat engine?
thermal power station
internal combustion engine
steam train.
How do you get more work out of a system?
You need a larger difference between the heat going into the heat engine than the heat leaving (bigger QH than QL)
How do you calculate work from a heat engine?

How do you calculate the heat engine’s efficiency?
An amount of heat, QH is supplied from the hot reservoir to the engine in each cycle. Of that heat, some is output as work and the rest, QL is given off as waste heat to the cold reservoir.

How do you calculate power of a heat engine?
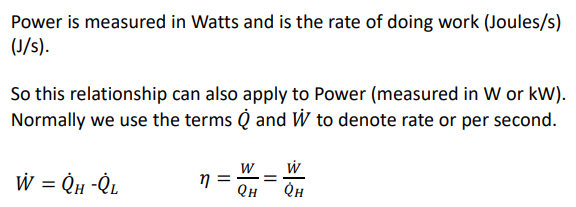
What is a reversible process?
process in which the system and surroundings can be restored to the initial state from the final state without producing any changes in the thermodynamics properties of the universe
At the end of a forwards and backwards reversible process, both system and surroundings are returned to their initial states
What is an irreversible process?
In the irreversible process the initial state of the system and surroundings cannot be restored from the final state. Examples of irreversibility sources: Heat transfer through a finite temperature difference Friction Fast expansion or compression of a fluid
Are reversible processes possible?
In reality, reversible processes can not exist, because it is not physically possible to have processes occur so infinitesimally slowly that they are quasi-static and in constant equilibrium. In reality, all processes are irreversible, but looking at reversible cycles allows us to determine the best possible system.
For mechanical work: How do you calculate work done?
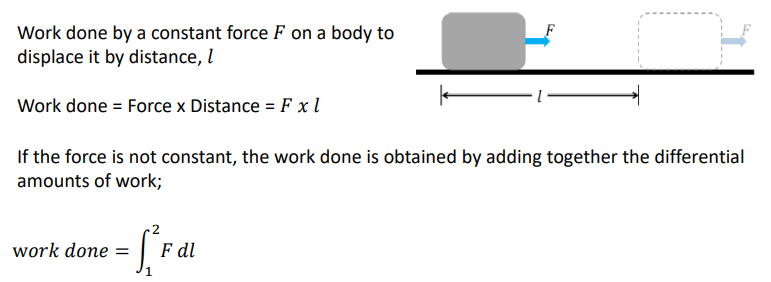
What are the requirements for a work interaction between a system and the surroundings?
1. There must be a force acting on the boundary
2. The boundary must move
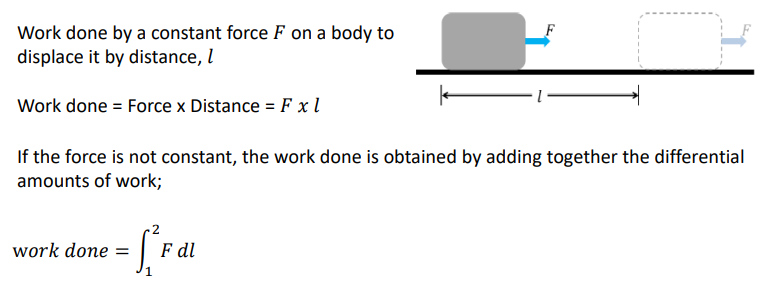
Moving Piston example
JUST AN EXAMPLE:
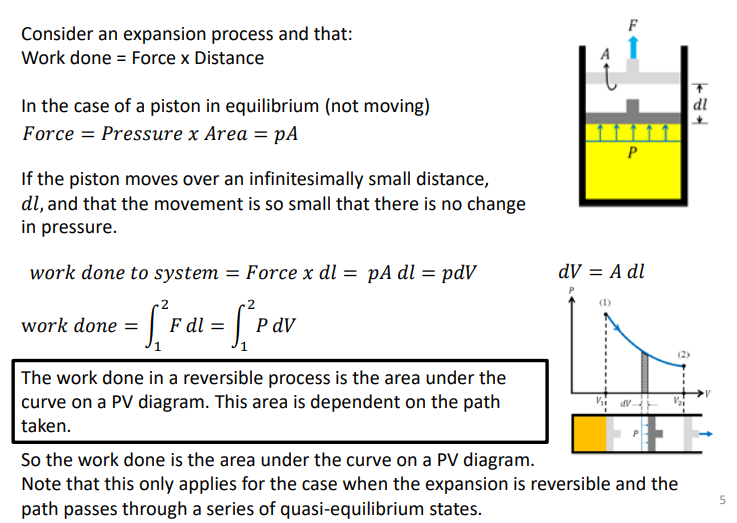
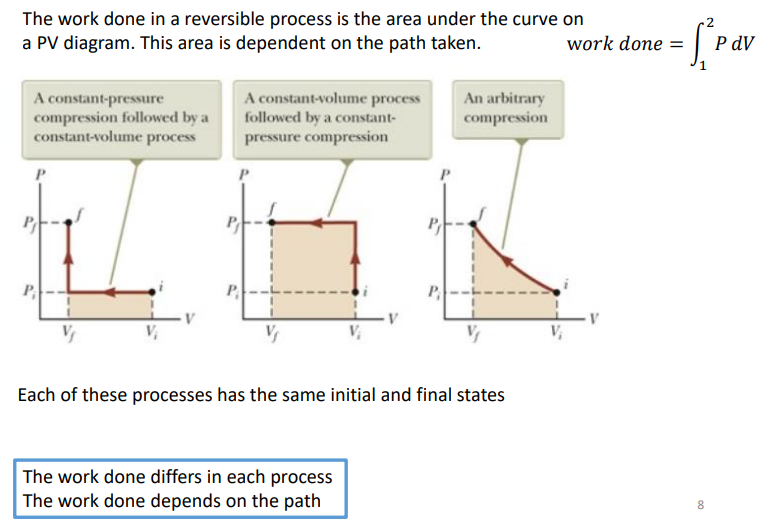
How do you calculate the work done on a reversible process of a P-V diagram?
The work done in a reversible process is the area under the curve on a PV diagram. This area is dependent on the path taken
what shape is a isothermal compression or expansion process on a P-V diagram?
Hyperbola - paths for isothermal processes are called isotherms.
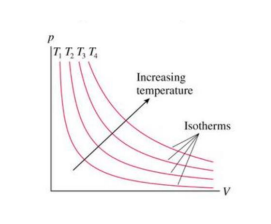
What is the relation between adiabatic and polytrophic processes
An adiabatic process is a particular type of polytrophic process in which there is no heat transfer
What is a polytrophic process?
A process where P V and T all vary
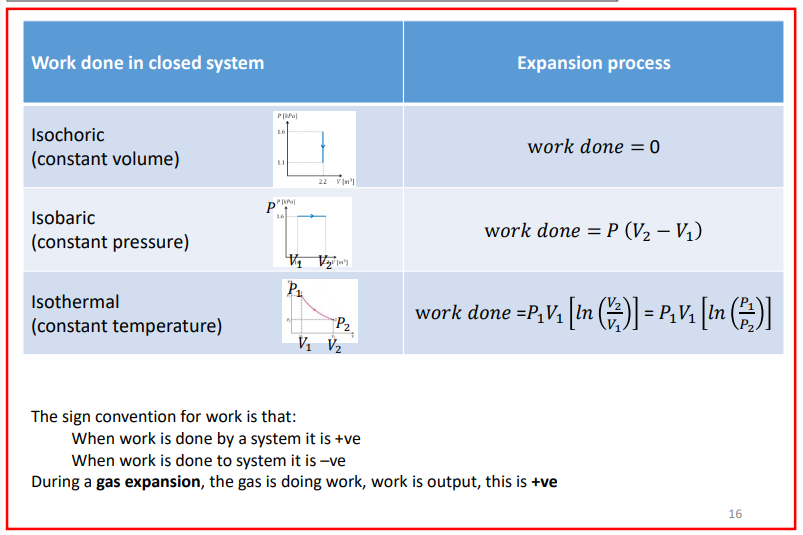
Work done in a closed system summary
JUST AS A REFERENCE
What is Specific Heat Capacity?
The Specific Heat Capacity describes how much heat energy it takes to raise the temperature of 1 kg of a given system by 1 degree centigrade (or 1 Kelvin)
What is Heat Capacity?
Heat Capacity describes how much heat energy it takes to raise the temperature of a given system by a given amount.
What is the formula for specific heat capacity?
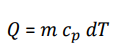
Name some Heat Capacity applications
Substances having a small specific heat capacity, are very useful as material in cooking instruments such as frying pans, pots, kettles and so on, because, they can be quickly heated up even when small amount of heat is supplied.
Sensitive thermometers also must be made from materials with small specific heat capacity so that it can detect and show a change of temperature rapidly and accurately.
Substances that have a high specific heat capacity is suitable as a material for constructing kettle handles, insulators and oven covers, because, a high amount of heat will cause only a small change in temperature - the material won't get hot too fast!
Heat storage instruments are very useful and they are usually made of substances with a high specific heat capacity because the temperature of the substances will reduce at a slower rate
Water is excellent as a cooling agent in engines. This is because the high specific heat capacity means that it can absorb a large amount of heat whilst the increase in temperature is relatively small.
Water is also used to heat houses because as it is heated up it tends to retain heat and warm the house due to its high specific heat capacity
How do you calculate power using an adaptation of the specific heat capacity equation?
Power = energy/time —> measured in J/s or W
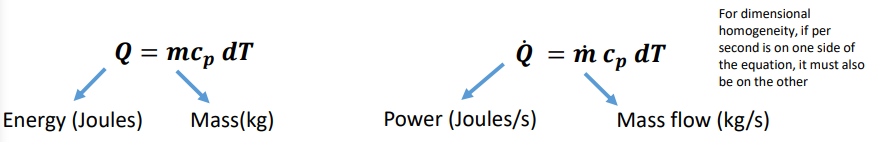
What are the three key equations for constant volume, constant pressure or solids and liquids?

What is a state function?
A property that is only dependent on the current state of the system. A state function does not depend on the path that was taken to reach the current state.
Examples of state functions include:
U Internal energy
H Enthalpy
S Entropy
P Pressure
T Temperature
V Volume
What does it mean to be specific in terms of state functions?
A state function can be given per kg – in this case it is know as ‘specific’ and is denoted with a lower case letter:
u specific internal energy
h specific enthalpy
s specific entropy
v specific volume
What is the internal energy (U) of a substance?
All of the kinetic and potential energy of the system.
How do you calculate the change in internal energy? (delta U)
If the system undergoes a heat addition process, the change in internal energy is equal to the heat added.
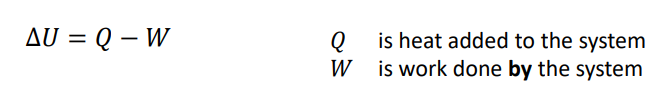
What is the first law for a closed system?
The first law states that the change in internal energy of a system is equal to the heat transferred to the system minus the work done by the system.
The energy balance is maintained within the system
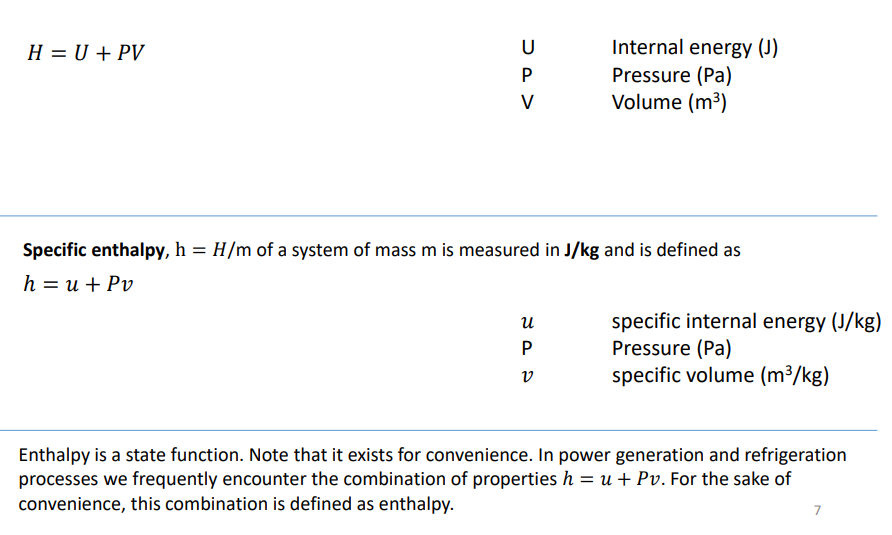
What is enthalpy?
Enthalpy is a measure of the energy in a thermodynamic system. It is equivalent to the total heat in a system and is measured in J
How do you calculate enthalpy and specific enthalpy (using internal energy, pressure and volume)?
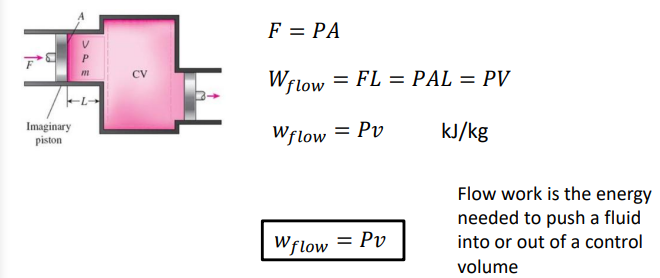
What is flow work?
Flow work is the work that is required to push the mass into or out of the control volume as open systems (unlike closed systems) involve mass flow across their boundaries.
How do you calculate workflow?
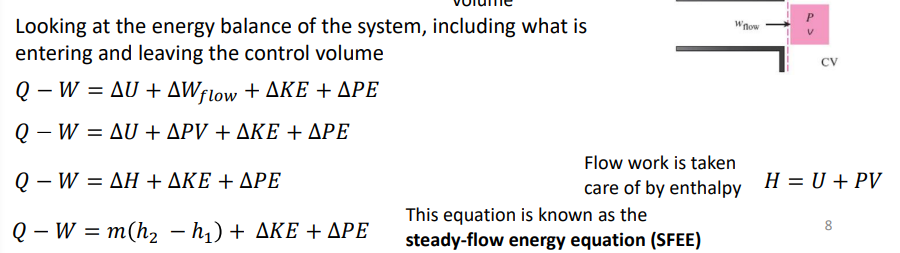
What are the steady-flow energy equation?
What is a heat engine?
A system which converts heat energy (𝑄 subscript𝐻) to mechanical energy which can be used to do work (𝑊). During this process some of the heat is lost to the surroundings and some energy is unusable because of friction (𝑄𝐿 ).
A heat engine includes a number of components. We can consider these components as a single system. Because the heat engine operates in a cyclical manner (a number of processes which are continually repeated), we can represent the heat engine schematically as shown.
How can you represent a heat engine in a diagram form?
The heat flow from the high temperature reservoir is known as 𝑸𝑯 The heat flow to the low temperature reservoir is known as 𝑸𝑳 The work output is 𝑾 The temperature of the high temperature reservoir is known as 𝑻𝑯 The temperature of the low temperature reservoir is known as 𝑻𝑳
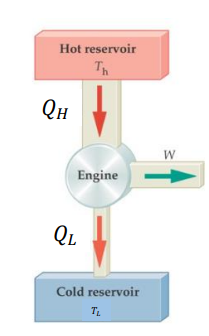
What is an example of a heat engine?
Thermal power station, internal combustion engine, steam train
How do you calculate the work done of a heat engine?
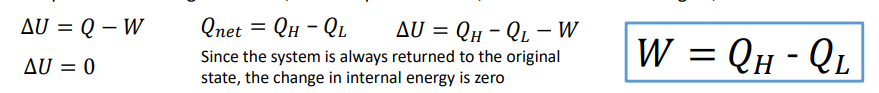
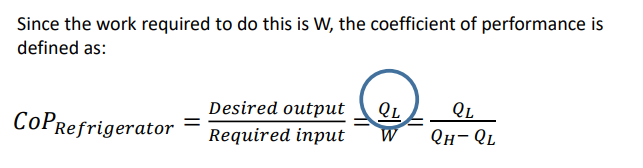
What is a refrigerator?
A refrigerator is a reversed heat engine which cools and maintains the temperature of a body lower than atmospheric temperature. We want cooling input, this is achieved by work input.
What is an example of a refrigeration?
Household refrigerator Cooling in AC systems Battery cooling in electric vehicle
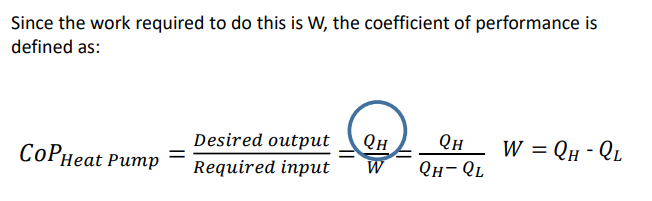
What us a heat pump?
A heat pump operates in the same way as a refrigerator, but the hot and cold reservoir temperatures are different. For a refrigerator, the hot reservoir is atmospheric temperature, and the cold reservoir is at a temperature below this. For a heat pump, atmospheric temperature is the cold reservoir and the hot reservoir is at a temperature above this. We want heat output, this is achieved by work input.
What is an example of a heat pump?
Home heat pump to provide home heating
Automotive heat pump to provide cabin warming in electric cars
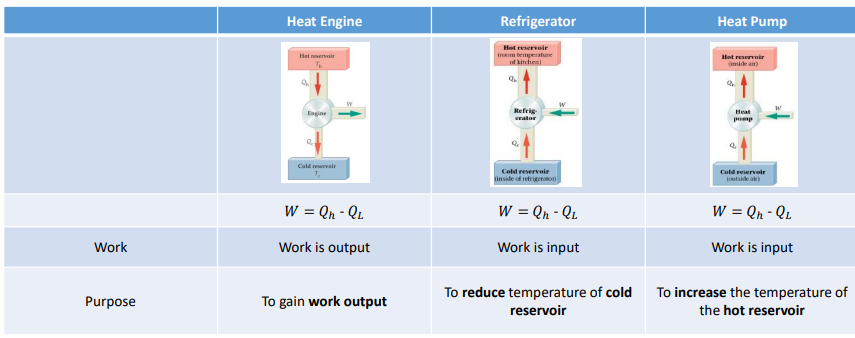
Compare the work equation, whether the work is an in out or output and the purpose of each if the following systems: heat engines, refrigerator and heat pump
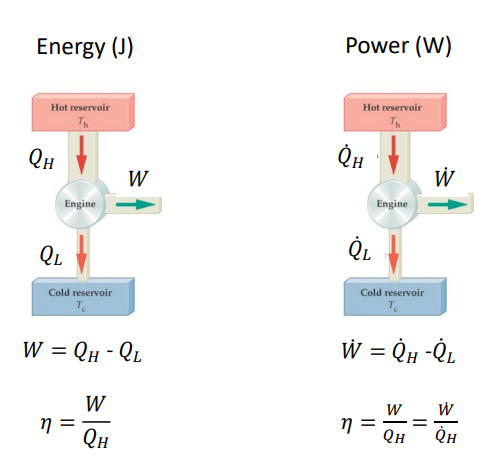
What is the efficiency of a heat engine? And what is the equation?
An amount of heat, QH is supplied from the hot reservoir to the engine in each cycle. Of that heat, some is output as work and the rest, QL is given off as waste heat to the cold reservoir.
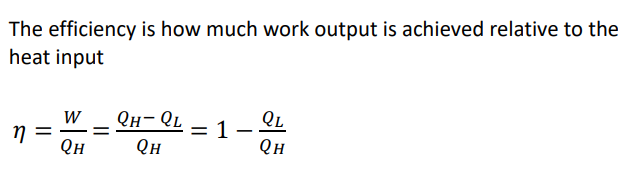
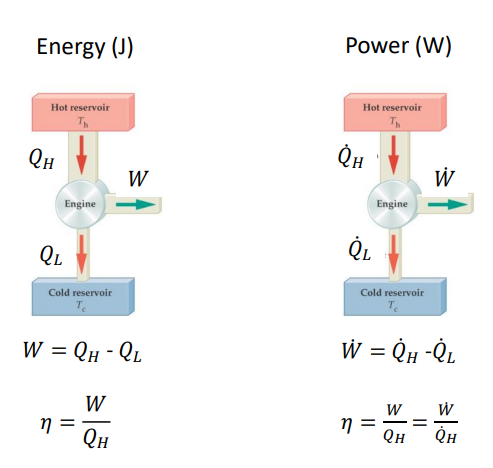
What is the efficiency of the POWER of a heat engine? And what is the equation?
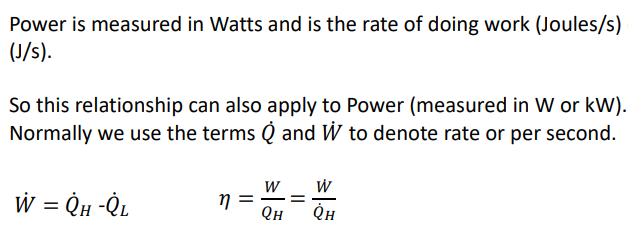
What is the coefficient of performance?
The Coefficient of Performance (CoP) is a measure of the useful heating or cooling to the amount of work input for REFRIGERATORS and HEAT PUMPS
CoP is a measure of performance, but is not measured as a percentage. Normally, the value for CoP will be more than 1.
It is a useful measure of how effective a device is at giving a desired output in relation to the required input.
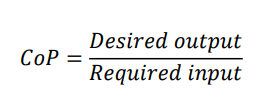
What is equation for the CoP for a refrigerator involving the work equation?
What is equation for the CoP for a refrigerator involving the work equation?
What is sensible heat?
Sensible heat is the energy associated with a change in temperature without a phase change (eg change from liquid to vapour).
What is latent heat?
Latent heat is the energy associated with a change of state (solid to liquid or liquid to vapour)
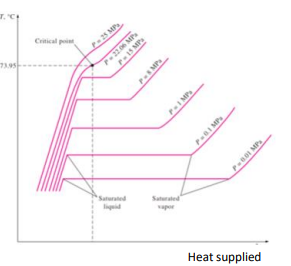
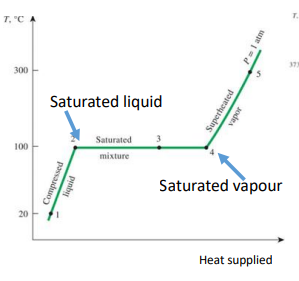
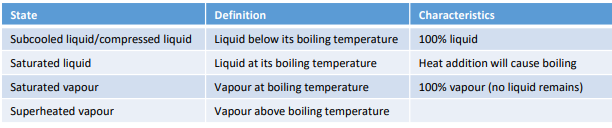
Describe the changing states graph with the following terminology: condensed liquid, saturated liquid, saturated mixture, saturated vapor, superheated vapor
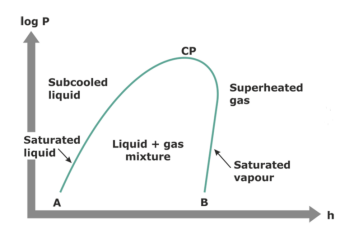
Describe the pressure-enthalpy diagram with the following terminology: saturated liquid, subcooled liquid, liquid + gas mixture, superheated gas, saturated vapor
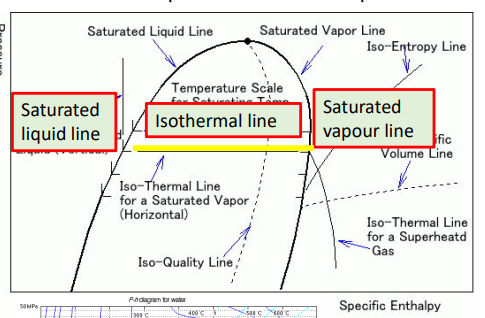
What happens in a process which follows a horizontal line between the saturated liquid and saturated vapor lines?
If the process is moving from left to right, the substance is increasing in enthalpy (gaining energy), yet the temperature is constant. This is because the energy is used as latent heat to break bonds as the saturated liquid gradually vapourises until it becomes saturated vapour.
If the process is moving from right to left, the substance is decreasing in enthalpy (losing energy), yet the temperature is constant. This is because the energy is used as latent heat to make bonds as the saturated vapour gradually condenses until it becomes saturated liquid
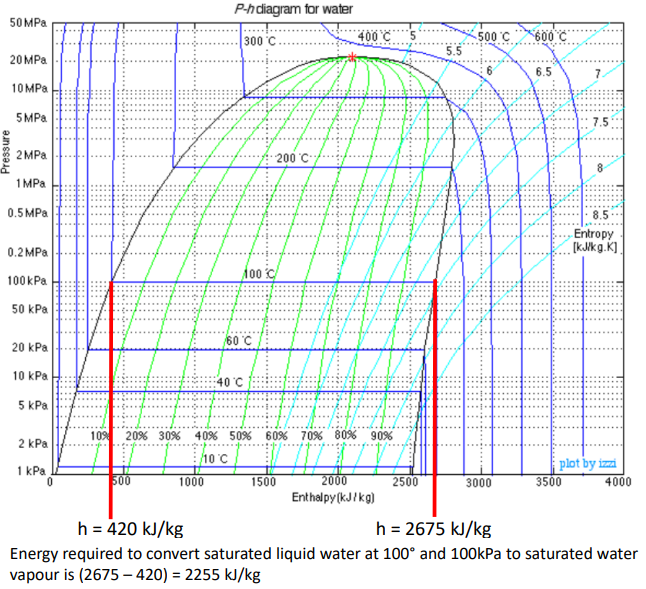
What do the coloured lines on this diagram represent?
black - lines of constant temperature
green - lines of constant entropy
mint - lines of constant quality (proportion from saturated liquid to saturated vapour)